QIAL is engaged in developing state-of-the-art spectral CT instrumentation, algorithms and imaging methods. Spectral CT can be performed using either two scans with energy integrating detectors (as with dual energy CT systems) or using photon counting detectors (PCD).
We have developed a dual tube/detector micro-CT system and used it with nanoparticle-based contrast agents for theranostics (i.e. therapy and diagnostics) such as iodinated liposomes and gold nanoparticles in various cancer and cardiac applications. We were the first to demonstrate dual energy CT imaging using dual nanoparticles (gold and iodine) to image two important vascular biomarkers in tumors—the microvascular blood volume and vascular permeability. Dual energy CT enabled separation of iodine and calcium or iodine and gold, and it was applied by us in imaging myocardial infarction, classification of atherosclerotic plaque composition, and the classification of tumor aggressiveness in classification of lung cancer, and classification of radiation therapy response in primary sarcoma tumors. On contrast agents we collaborate with Drs. Ketan Ghaghada and Jennifer West.
Photon-counting detectors are a promising new technology for CT. They provide energy-resolved CT data at very high spatial resolution without electronic noise and with improved tissue contrasts. We have now developed two preclinical imaging systems using photon counting detectors and applied them for performing spectral CT of tumors and the beating murine heart. The systems are described next.
I). Dual Source Hybrid (EID/PCD) Micro-CT: This unique state-of-the-art dual tube/detector micro-CT system (see Fig. E1) was built by us at Duke to address such challenging tasks such as multi-energy CT but also dynamic imaging as for cardiac and perfusion studies. One of the detectors is energy integrating (EID) and the other is photon counting.
Fig. E1: (A) A photo of the dual source hybrid micro-CT system in a rotating specimen geometry. X-ray tubes and the detectors are arranged orthogonally. The system uses:
· 2 Varian G297 x-ray tubes (fs=0.3/0.8 mm)
· 2 Epsilon High Frequency x-ray generators (EMD Technologies (Quebec, Canada)
· A Dexela EID with a CsI phosphor and 75-µm pixel size.
· A SANTIS 1604 CdTe-based PCD from Dectris, Ltd. (www.dectris.com). This detector has 150-μm pixel size, 16×4 cm field of view and four energy thresholds.
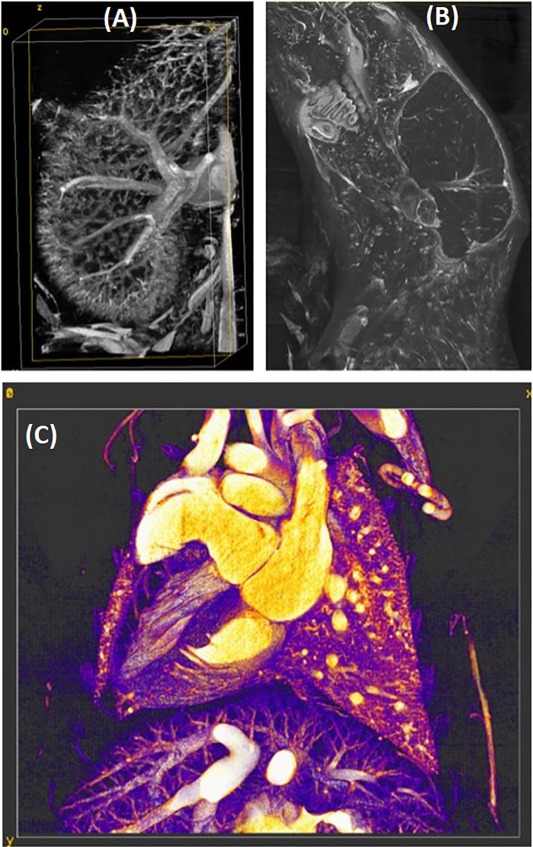
(B) Reconstructions at 125 microns of an in vivo scan of a sarcoma tumor-bearing mouse injected with liposomal iodine accumulated and gold nanoparticles contrast agents with vials of I (15 mg/mL), Gd (15 mg/mL), water, Ca, and Au (8 mg/mL). The same axial slice obtained with the hybrid iterative reconstruction is shown for all PCD energies and the EID. The yellow ellipsoid indicates the tumor.
C) Material decomposition of a single slice and a volume rendering show the separation of iodine (red), gold (green), gadolinium (blue), and calcium (white). Iodine has accumulated in the tumor and the gold is seen in the vasculature.
II) A Single Source PCD-Micro-CT System for High Resolution ex vivo Imaging

Fig. E2: (A) A photo of the PCD-CT system. It uses a XC-Thor a CdTe-based PCD from Direct Conversion (https://directconversion.com ) with anti-coincidence correction. This detector offers the potential for high-resolution imaging (1024 x 256 pixels, 100-μm pixel size) but has only 2 energy thresholds.
X-rays are generated by a L9181-02 39W micro-focus X-ray source (Hamamatsu). For tomographic scans we use a motorized rotator (Newport, model URS 100BPP) to slowly rotate the object (mouse/phantom) positioned in a 3D printed cradle during acquisition.
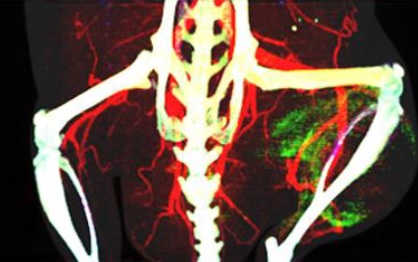
(B) A volume rendered dataset of a mouse with a sarcoma tumor (ellipsoid) imaged ex vivo with an isotropic resolution of 38 microns. We have perfused the mouse using a Barium (Ba) based contrast agent (BriteVu, scarletimaging.com). The two energy thresholds (30, 38 keV) of the PCD enabled the separation of Barium in the vasculature from Calcium (bone). The scanning parameters were 80 kVp X-ray , 200 microA, 2 secs/projection and 900 projections. The scan took 2 hours to complete.
Cardiac Photon Counting CT: We have demonstrated the power of spectral and temporal synergy and our complementary reconstruction techniques, performing 5D cardiac PCD-CT data acquisition and reconstruction in a mouse model of atherosclerosis, including calcified plaque.

We presented this talk on spectral micro-CT and its applications at the NCI CIRP Annual Meeting, June 22-23, 2022.
|
|