Brad’s JACS 2015 paper about the peptide rescue of GG-motif mutations was selected for the JACS young investigator virtual issue. In this paper, we reported that some of the mutations in MoaA that cause human Moco deficiency disease could be rescued by a synthetic peptide, suggesting potentials for the future development of a novel therapeutics. Interestingly, this study also revealed that the C-terminal tail of MoaA is involved in the radical initiation during the MoaA catalysis.







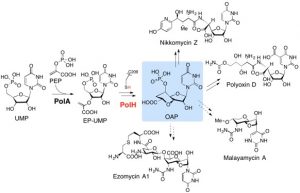


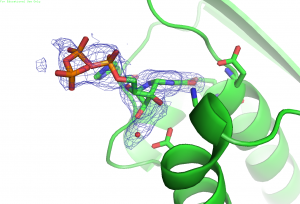 We are interested in the functions and mechanisms of enzymes involved in the biosynthesis of natural products and cofactors as well as those in fungal cell wall biosynthesis. The target enzymes play essential roles in inheritable human disease, or bacterial or fungal infectious disease. We aim to understand the mechanisms of these enzymes and use the knowledge to discover novel therapeutics. Specifically, we are interested in:
We are interested in the functions and mechanisms of enzymes involved in the biosynthesis of natural products and cofactors as well as those in fungal cell wall biosynthesis. The target enzymes play essential roles in inheritable human disease, or bacterial or fungal infectious disease. We aim to understand the mechanisms of these enzymes and use the knowledge to discover novel therapeutics. Specifically, we are interested in: