The incidence of fungal infection has been increasing in the past decade, but our current treatment option is limited due to the toxic side effects of the existing molecules and increasing drug resistance. Inhibitors of fungal cell wall biosynthesis have been found in Nature, and exhibit highly potent and selective antifungal activities. While some of these antifungal molecules have been successfully used in clinics, many others have not been clinically exploited. We are studying those molecules with the long-term goal of providing the novel and clinically useful antifungal agents.

Our current focus is the biosynthesis of the peptidyl nucleosides (PN) antifungals that inhibit chitin synthase. PNs inhibit chitin synthase, enzymes essential for fungal cell wall formation, and provide potent in vivo activities against some of the pathogenic fungi. These molecules are also synergistic with the clinically used antifungals, such as echinocandins. Therefore, the development of these molecules will strengthen our current antifungal chemotherapy. To provide novel chitin synthase inhibitors, we are taking chemoenzymatic and biosynthetic approaches to diversify the drug discovery efforts.
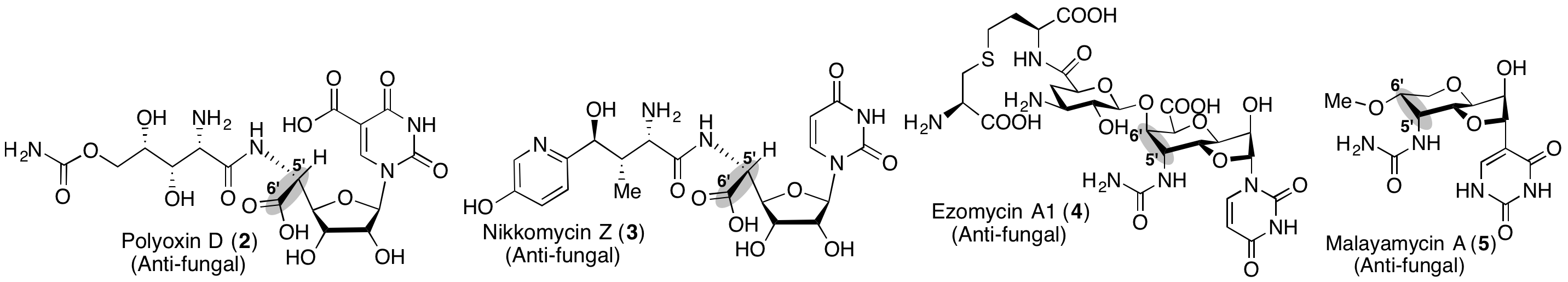
In our biosynthetic study, we discovered a radical SAM (S-adenosylmethionine) enzyme, PolH, that catalyzes the key C-C bond forming reaction using a unique free radical-mediated mechanism (
Nat. Chem. Biol. 2016). Subsequently, we discovered that the following biosynthetic steps require a “cryptic phosphorylation” at the 2′-position. Consequently, we have successfully revealed the functions of all the enzymes required for the nikkomycin and polyoxin biosynthesis. We also characterized other antifungal nucleosides and proposed that these molecules are biosynthesized through conserved pathways that diverge later to create structural diversity. See
Nat. Chem. Biol. 2021 and
ChemBioChem 2023.Future directions:
• Metallo-enzymology: The study also revealed several functionally novel metallo-enzymes. We are also studying the mechanisms and structures of those enzymes and their homologs.
• Genome mining: Our biosynthetic studies so far suggested a significant potential of the genome mining discovery of novel nucleoside antimicrobials. We are currently testing such hypothesis by heterologous expression.
• Chemoenzymatic synthesis of unnatural nucleoside antifungals: Together with our study on fungal cell wall biosynthetic enzymes, we are currently performing structure-guided development of novel antifungal nucleosides. In this project, we combine biosynthetic enzymes and chemical synthesis to prepare unnatural analogs.




