Current projects
We are broadly interested in understanding the functions and mechanisms of enzymes that are relevant to human health. In particular, we currently study enzymes in antifungal natural product biosynthesis, fungal cell wall biosynthesis, and bacterial and human cofactor biosynthesis. We also study the functions and mechanisms of metalloenzymes, such as radical SAM enzymes. For these diverse projects, we use a combination of approaches from organic chemistry, biochemistry, molecular biology, and spectroscopy (see Experimental Techniques for details).
(1) Radical SAM enzymology
One of our foci is the functions and mechanisms of a group of enzymes called radical S-adenosylmethionine (SAM) enzymes, one of the largest groups of enzymes with > 113,000 functional domains. These enzymes catalyze the reductive cleavage of S-adenosyl-L-methionine (SAM) to generate transient 5’-deoxyadenosyl radical, which is subsequently used to catalyze various free radical-mediated reactions (see Figure below). Many of these enzymes are found in biological processes closely associated with human diseases. Also, these enzymes catalyze free radical-mediated reactions, which until recently, were considered rare in enzyme catalysis. Thus, their functions and mechanisms form the foundation of a novel paradigm in enzymology. See the project page for details.
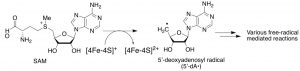
Reductive cleavage of SAM by radical SAM enzymes.

Natural products biosynthesized by radical-mediated mechanisms.
(2) Molybdenum cofactor biosynthesis
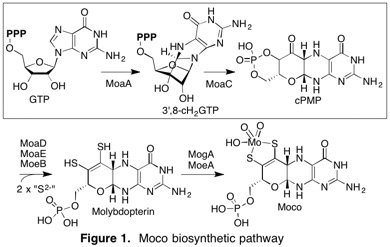
(3) Antifungal antibiotics and fungal cell wall biosynthesis.

Techniques
To address our biochemical questions described above, we take diverse approaches. Each student/postdoc in the lab will learn multiple of these techniques in excellent depth and combine them to address their scientific questions. Therefore, our studies are highly interdisciplinarity in nature. The skills and knowledge obtained from these studies are translatable to many other systems, providing an outstanding training opportunity for students and postdocs. See Experimental Techniques for details.