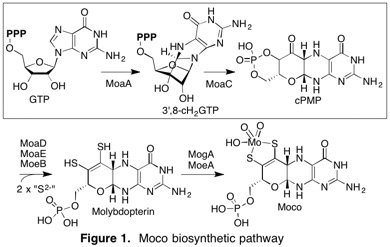
Molybdenum cofactor (Moco) is an enzyme cofactor found in almost all organisms from all kingdoms of life and plays central roles in various metabolic and catabolic pathways. Moco cannot be acquired from the environment and hence, must be biosynthesized de novo through a conserved pathway. Moco biosynthesis plays a vital role in various scientific contexts and is associated with multiple medical and environmental problems. For example, in humans, Moco is essential in various detoxification pathways, and perturbation in its biosynthesis causes a fatal and currently incurable disease. In bacteria, Moco plays a crucial role in virulence. Therefore, a fundamental understanding of Moco biosynthesis is necessary.
We recently discovered a novel biosynthetic intermediate (3’,8-cH2GTP) and revised the functions of two enzymes, MoaA and MoaC (Fig. 1; JACS 2013, 135, 7019-32; PNAS 2015, 112, 6347-52.). We are now characterizing the mechanisms of these enzymes with the following specific questions.
• How does MoaA control the highly reactive free radicals to produce the structurally unique cyclic nucleotide, 3′,8-cH2GTP? (see JACS 2020)
• How does MoaC catalyze a complex rearrangement of 3′,8-cH2GTP into cPMP? How does the protein conformational dynamics play roles in this process? (see PNAS 2015 and Biochemistry 2015)
• How do mutations found in human Moco deficiency patients affect the functions of MoaA and MoaC? Can we rescue the mutations using small molecules? (see JACS 2015)
We are tackling these questions using multidisciplinary approaches, including enzymology, organic chemistry, NMR, EPR, and X-ray crystallography.
The project will be developed in the future to understand the later steps of the pathway involving sulfur trafficking and metabolisms, relevant to cellular signaling in humans and infectious disease in bacteria.

