During the autooxidation of dopamine1, dopamine loses an electron (e-) from each of the two OH groups on the ring to form a compound called a “quinone” (see Figure 4).
Figure 4 Dopamine is oxidized by O2 alone to form a quinone and the superoxide radical. This can occur in the synaptic space or inside the terminal.
Oxygen picks up the e- (2 molecules of O2 picked up an e- each) leaving it with an extra unpaired e- (it now has 13 e- instead of 12 in the outer orbital). This is a very unstable form of oxygen called a superoxide radical (O2*-)(please note – the radical is usually indicated by a dot, not an asterisk). This oxygen radical is a type of free radical2. Free radicals have a lone electron in their outer electron orbital (Figure 5) and they are very reactive molecules because they tend to donate a single e- or steal one from another molecule. Free radicals often have a *- shown to indicate the lone e-.
Figure 5 The superoxide radical contains 13 electrons in the outer orbitals of the 2 oxygen atoms. The extra electron gives the molecule a very reactive character.
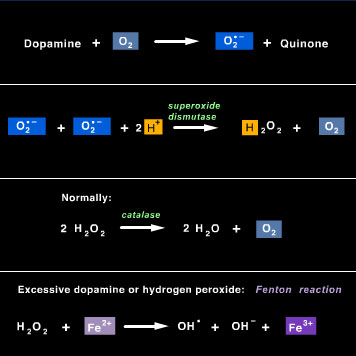
To keep the level of oxygen radicals3 low in our bodies, oxygen radicals are “scavenged” by enzymes4 to render them harmless. For example, superoxide radicals are reduced with the help of the enzyme superoxide dismutase4 to form hydrogen peroxide5 (H2O2), which is then converted (detoxified) by the enzyme catalase to water and O2 (see Figure 6 for the entire process). However, levels of oxygen radicals can rise, for example, after repeated or excessive use of drugs6 such as methamphetamine7, or during aging, when scavengers may be not as active. In this case, hydrogen peroxide becomes reduced by iron (Fe2+), which donates an electron to produce the hydroxyl radical (OH*), a very nasty molecule. The hydroxyl radical is extremely reactive, and it’s a great oxidizing agent8. The hydroxyl radical oxidizes cellular components such as lipids, proteins and DNA by literally stealing an e- (associated with an H atom) from them…resulting in damage to the cells.
Figure 6 Dopamine is oxidized by oxygen sequentially to form the superoxide radical, hydrogen peroxide (H2O2), and the hydroxyl radical. The hydroxyl radical is the most reactive and most damaging form of oxygen radicals.
Most recently, newer theories to explain the neurotoxicity of methamphetamine have emerged. These involve effects of methamphetamine on the dopamine transporter9 to disrupt ion10 homeostatis. This will not be discussed here.
Definitions:
1 a neurotransmitter stored in vesicles of nerve terminals; it is a monoamine that is easily oxidized. This neurotransmitter is contained in neuron pathways important in brain stimulation, addiction and control of movement.
2 an atomic or molecular species in which 1 or more outer orbital(s) contains an unpaired (lone) electron.
3 the reduction of oxygen by sequentially gaining electrons yields oxygen radicals such as the superoxide (O2*-) and the hydroxyl (OH*) radical. It is the OH* that damages lipids, proteins and DNA.
4 the enzyme that reduces the superoxide radical (O2*-) to hydrogen peroxide (H2O2). This enzyme diminishes with aging and in some neurodegenerative disorders like Alzheimer’s disease.
5 formed from the reduction of the superoxide radical by superoxide dismutase, but it is not actually a radical. It is detoxified by conversion to O2 and H2O by the enzyme catalase.
6 a substance that affects the structure or function of a cell or organism.
7 a derivative of amphetamine that increases the release of dopamine into the synaptic space. It causes increased alertness, restlessness and agitation.
8 a species that is good at gaining electrons (e.g. O2, Fe3+)
9 a protein that usually exists within a membrane to transport a compound (either large or charged) across the membrane to the other side.
10 an atom, radical, or molecule that has gained or lost one or more electrons. Therefore it acquires a net negative or positive charge.