Our lab looks at the design of membrane transport proteins—how they use their architecture to take advantage of selective transport and how we can use this selective transport for therapeutic potential in humans.
About Membrane Transport Proteins
Membrane transport proteins can selectively recognize a variety of substrates of differing sizes and physicochemical properties for cellular transport, thus they are responsible for the movement of key molecules and the transfer of information across cell membranes—events central to many important physiological processes.
Our Techniques
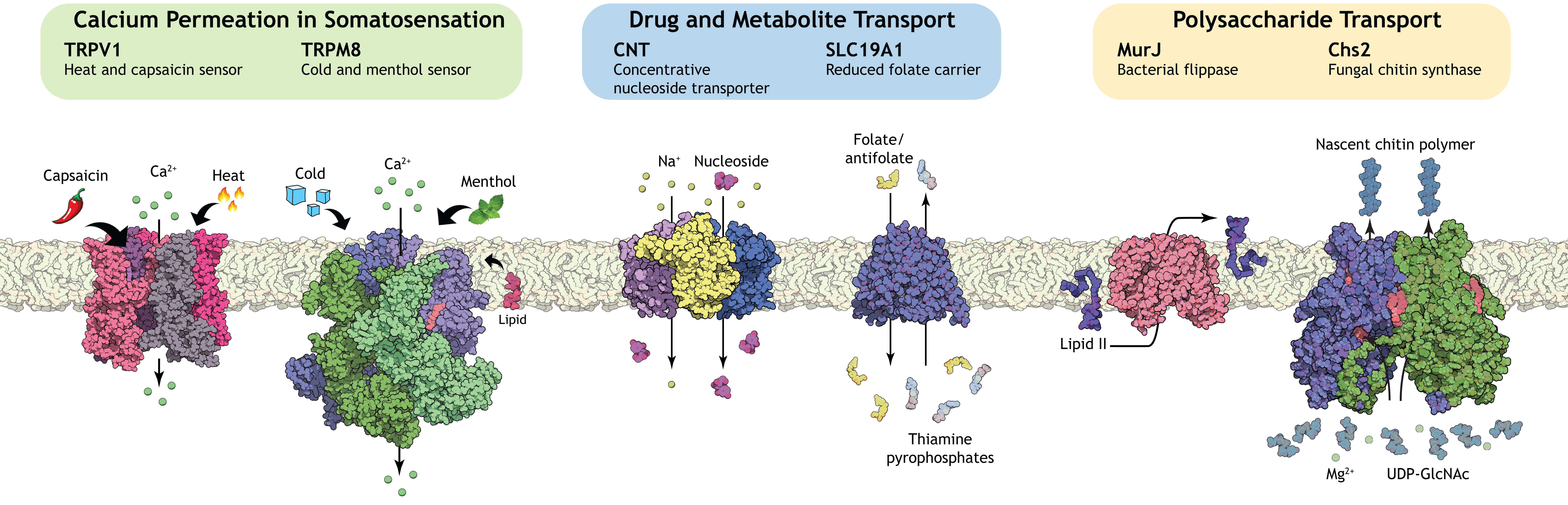
We utilize a variety of structural and biophysical methods—including cryo-EM, electrophysiology, isothermal titration calorimetry, radioligand flux and binding assays, and UV-vis/fluorescence-based spectroscopic assays—to study three main transport processes: ions (calcium), nucleic acids, nutrients, and pharmaceuticals (nucleosides, folate, and related chemotherapeutics), and cell wall precursors (peptidoglycan and chitin synthesis). Studying these systems probes the physiological and pathophysiological processes underlying somatosensation, pain, cancer, and bacterial/fungal infection.
Areas of Research Focus
Calcium Permeation in Somatosensation
In the Lee lab, we focus on elucidating the molecular mechanisms of the large superfamily of integral membrane proteins known as transient receptor potential (TRP) channels. These channels form the basis for various forms of mammalian sensation, being activated by both noxious physical (hot/cold, pain) and chemical (menthol, wasabi) stimuli.
Drug and Metabolite Transport
Our lab is interested in a detailed mechanistic understanding of the cellular uptake of drugs and metabolites/nutrients by solute carriers (SLCs), as well as the often inevitable drug-drug and drug-nutrient interactions that occur. Specifically, we are interested in nucleoside and nucleoside-derived drug transport mediated by Concentrative and Equilibrative Nucleoside Transport proteins (CNTs/ENTs), and folate and anti-folate drug transport by the Reduced Folate Carrier (RFC). These transporters are vital to a wide variety of physiological processes including metabolism, cellular signaling, and drug uptake and excretion.
Polysaccharide Transport in Microbial Cell Wall Synthesis
The biosynthesis of many important polysaccharides (including peptidoglycan, lipopolysaccharide, chitin, and N-linked glycans) necessitates the membrane transport of polysaccharide precursors from their cytoplasmic site of synthesis to their site of assembly outside the cytoplasm. To accomplish this task, cells utilize two types of systems. Fungi utilize synthases simultaneously polymerize precursors into long extracellular polymers and transport (chitin) while bacteria utilize lipid-linked transport systems where translocases attach the precursor to a carrier lipid, then a flippase transports the lipid-linked precursor across the membrane. We want to understand these mechanisms of transport by membrane transport proteins and how inhibitors block these systems from exerting their cellular effects.