Reactive oxygen species (ROS) are molecules containing an oxygen atom with an unpaired electron in its outer shell. As ROS are formed, they become very unstable due to the unpaired electron now residing in the outermost shell. The unstable forms of oxygen are sometimes called free radicals.
How do ROS actually get generated in cells? One way is via cellular respiration driven by the electron transport chain in the mitochondria. The electron transport chain is responsible for generating ATP, the main source of energy for a cell to function. A key molecule that helps “jump start” the electron transport chain, is NADH (or nicotinamide adenine dinucleotide), which serves as the electron donor (i.e., the H in the NADH). NADH is often referred to as a “coenzyme”, even though it is not an enzyme (a protein).
NADH is present in all cells–it is generated by many biochemical reactions. One way that NADH gets generated in large quantities is when alcohol is metabolized (or oxidized) to form acetaldehyde and then to acetic acid. During the metabolism of alcohol, the enzyme alcohol dehydrogenase (ADH) and NAD+ convert alcohol to acetylaldehyde, generating NADH. A second enzyme, aldehyde dehydrogenase (ALDH) and NAD+ convert acetaldehyde to acetic acid, generating even more NADH. In these reactions, the coenzyme NAD+ is reduced to NADH (and alcohol and acetaldehyde are oxidized).
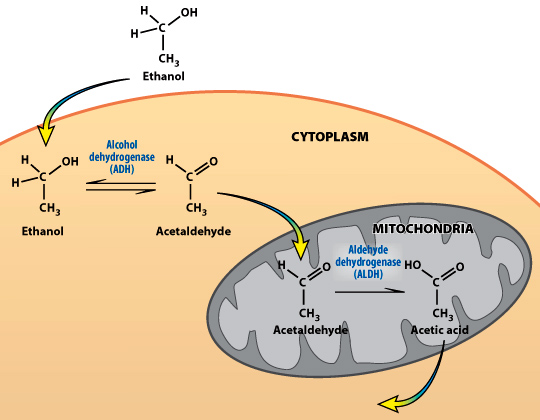
Review the oxidation of alcohol by alcohol dehydrogenase (ADH)
To learn more about the oxidation of alcohol by ADH, you can watch a virtual reality game called “DiVE into Alcohol” at sites.duke.edu/rise/dive-into-alcohol
Now there is plenty of NADH available to “jump start” mitochondrial respiration. NADH moves from the cytosol into the mitochondria where it donates an electron to the electron transport chain. The electron transport chain consists of a group of proteins (and some lipids) that work together to pass electrons “down the line”. Finally in the presence of oxygen, ATP is formed, providing energy for many cellular functions.
However, some electrons can “escape” the electron transport chain and combine with oxygen to form a very unstable form of oxygen called a superoxide radical (O2•-). The superoxide radical is one of the reactive oxygen species (ROS).
The superoxide radical is a type of free radical. Free radicals have a lone electron in their outer electron orbital and they are very reactive molecules because they tend to donate single electrons (e-) or steal e- from other molecules. Free radicals can be destructive to cellular components. Free radicals often have a • shown to indicate the lone e-.
Our cells have ways to protect themselves from the damaging effects of these reactive molecules. For example, our cells are able to maintain low levels of the superoxide radicals with the help of the enzyme superoxide dismutase (SOD). SOD helps reduce superoxide to form hydrogen peroxide (H2O2), which is then converted (detoxified) by the enzyme catalase to water and O2.
However, sometimes the levels of superoxide rise, for example after alcohol exposure (which generates a lot of NADH). Thus, more hydrogen peroxide is formed and can’t be detoxified by the limited amount of catalase. Instead hydrogen peroxide becomes reduced by iron (Fe2+) (normally present in cells), which donates an electron to produce the hydroxyl radical (•OH), a very nasty molecule. It is extremely reactive, and it’s a great oxidizing agent. The hydroxyl radical oxidizes cellular components such as lipids, proteins, and DNA by literally stealing an e- (associated with an H atom) from them, damaging cells.
 Figure: The metabolism (i.e., oxidation) of alcohol produces NADH, which acts as an electron donor for the electron transport chain (molecules designated with roman numerals). Electrons (e-) that “leak out” of the electron transport chain (stars at I and III) combine with oxygen to produce superoxide radicals (O2•-). Through a series of reactions the superoxide radicals generate hydroxyl (OH•) radicals. Oxygen radicals are circled in red.
Figure: The metabolism (i.e., oxidation) of alcohol produces NADH, which acts as an electron donor for the electron transport chain (molecules designated with roman numerals). Electrons (e-) that “leak out” of the electron transport chain (stars at I and III) combine with oxygen to produce superoxide radicals (O2•-). Through a series of reactions the superoxide radicals generate hydroxyl (OH•) radicals. Oxygen radicals are circled in red.
To learn more how oxygen radicals oxidize lipids, proteins, and DNA to cause cellular destruction go to Module 3 at sites.duke.edu/thepepproject/.