The original version of the Breathalyzer™ included a mouthpiece and two chambers containing liquid connected to a meter that detects a change in color. To use the Breathalyzer™, the subject exhales through the mouthpiece into a test chamber filled with a reddish-orange solution of potassium dichromate (K2Cr2O7).
 Figure 4.7 An example of a newer version of a breath testing device
Figure 4.7 An example of a newer version of a breath testing device
In the Breathalyzer™, alcohol reacts with the reddish-orange potassium dichromate solution and turns green. The degree of the color change is directly related to the level of alcohol in the expelled air.
A photocell compares the difference in colors between the reacted mixture in the test chamber and a reference chamber containing unreacted mixture. The difference in colors produces an electrical current, which can be converted into a quantitative value for the BAC. Read on to understand how the chemical reaction produces the color change.
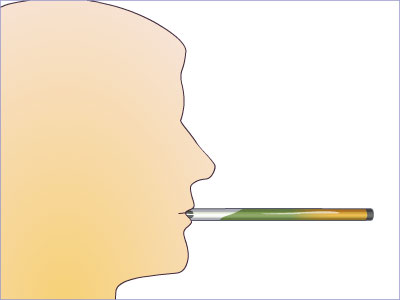 Figure 4.8 The ethanol vapor in the Breathalyzer™ triggers a chemical reaction with the compounds packed inside, turning the chemicals from orange to green. The more ethanol present, the greater the amount of green color is produced.
Figure 4.8 The ethanol vapor in the Breathalyzer™ triggers a chemical reaction with the compounds packed inside, turning the chemicals from orange to green. The more ethanol present, the greater the amount of green color is produced.
A description of different types of breath analyzers and how they work can be found at http://science.howstuffworks.com/breathalyzer3.htm