How do extracellular proteins control metal coordination chemistry?
Biological molecules can unlock and harness the special chemical power of metal ions, while also maintaining control of metal homeostasis. The Haas Lab investigates the interactions between metal ions and polypeptides, with a focus on the proteins that harness copper redox chemistry in human extracellular fluids and at membrane interfaces.
Bio-inorganic Chemistry and Human Copper Homeostasis
Did you know that metals play important roles in our health? You may have heard of some essential metals, like Ca, K, Fe, and Zn. And you probably know of some metals that are toxic, like Pb and Hg. Other metals can be used as medicines, like Pt drugs that treat cancer. Did you know that copper (Cu) is essential to your health? Cu is used in just about every cellular and systematic process including formation of connective tissues, cellular energy production (ATP), and neurotransmission.

One of the reasons copper is essential is because it has the ability to change oxidation states in biological environments. Cu can absorb and give off electrons by changing its oxidation state (its charge). The two most common oxidation states in biology are the +1 and +2 states. This changing in oxidation state is called redox chemistry and it allows copper to mediate some of the electron transfer reactions that are essential for things like cellular energy production (in the electron transport chain), antioxidant activity in cytoplasm, and in body fluids (in enzymes SOD 1 and SOD 3).
This redox chemistry is essential, however it can also be toxic if it is not controlled. If Cu is allowed to cycle through between its oxidation states unchecked, it can cause significant damage through oxidative stress. There is speculation and scientific data that suggest that malfunction of our bodies’ copper-handling proteins may result in neurodegenerative diseases like Alzheimer’s, Parkinson’s and prion diseases. The Haas Lab focuses on understanding how our proteins use copper for good while protecting against the bad.
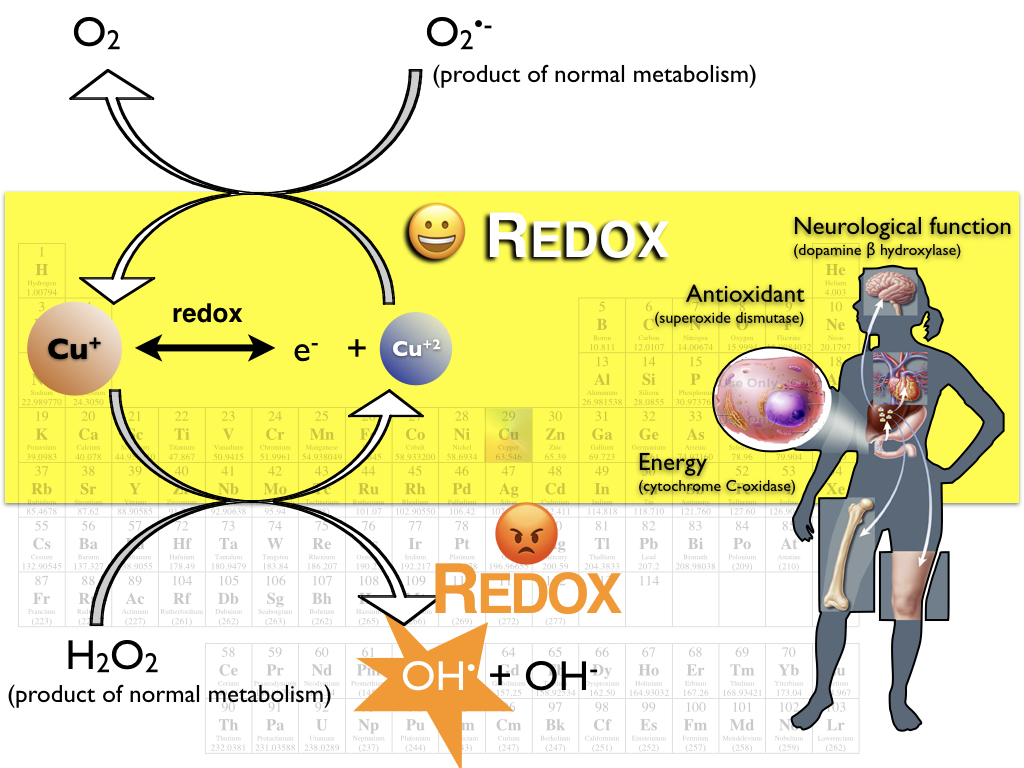
The Haas lab focuses on proteins that move Cu from place to place outside of, and between, cells in your body (in the extracellular environment). The proteins that do this must prevent redox cycling so that Cu con be transported without causing accidental damage to the molecules that carry them. Usually, proteins do this job, and they are called “Copper Chaperones”, and we believe that they prevent redox cycling by providing a very stable binding site for one copper oxidation state (and de-stabilizing the alternative copper oxidation state). Our main focus is on understanding how Cu is chaperoned by proteins in the extracellular spaces of human systems. We have published our work on Cu interactions with human serum albumin, amyloid beta (the peptide famous for its involvement in Alzheimer’s disease), and the human cellular copper transport protein 1 (Ctr1). And in collaboration with the Franz lab at Duke, we have also worked on human Histatin (an antibacterial protein in human saliva).

The Ctr1 protein allows Cu to pass safely through the membrane of your cells, but to do so, it must change its oxidation state. Ctr1 must be very good at allowing this Cu oxidation state change while protecting against bad redox chemistry. There is almost nothing known about how this happens: which other proteins are involved, what are the binding site geometries and ligands, where does the electron come from, how fast does it happen? We aim to find the answers to these questions through research!
Click on the video below to learn a bit about the Ctr1 protein!
[https://www.youtube.com/watch?v=ufiEZy1so_Y]
Projects
Below is a list of some of our projects and some related publications.
Cu shuttling in serum and synaptic spaces

- Schulte, N. B., Pushie, M. J., Martinez, A., Sendzik, M., Escobedo, M., Kuter, K., & Haas, K. L. (2023). “Exploration of the Potential Role of Serum Albumin in the Delivery of Cu(I) to Ctr1.” Inorganic Chemistry, 62(10), 4021–4034.
- Stefaniak, Ewelina, M Jake Pushie, Catherine Vaerewyck, David Corcelli, Chloe Griggs, Whitney Lewis, Emma Kelley, et al. “Exploration of the Potential Role for Aβ in Delivery of Extracellular Copper to Ctr1.” Inorganic Chemistry 59, no. 23 (December 2020): 16952–66. https://doi.org/10.1021/acs.inorgchem.0c02100.
- Stefaniak, Ewelina, Dawid Płonka, Simon C. Drew, Karolina Bossak-Ahmad, Kathryn L. Haas, M Jake Pushie, Peter Faller, Nina E. Wezynfeld, and Wojciech Bal. “The N-terminal 14-mer model peptide of human Ctr1 can collect Cu(ii) from albumin. Implications for copper uptake by Ctr1.” Metallomics : Integrated Biometal Science 10, no. 12 (December 2018): 1723–27. https://doi.org/10.1039/c8mt00274f.
Cu interactions with Amyloid Beta Peptides
- Pushie, M Jake, Ewelina Stefaniak, Madison R. Sendzik, Dimosthenis Sokaras, Thomas Kroll, and Kathryn L. Haas. “Using N-Terminal Coordination of Cu(II) and Ni(II) to Isolate the Coordination Environment of Cu(I) and Cu(II) Bound to His13 and His14 in Amyloid-β(4-16).” Inorganic Chemistry 58, no. 22 (November 2019): 15138–54. https://doi.org/10.1021/acs.inorgchem.9b01940.
Cu interactions with Human Serum Albumin
- Sendzik, Madison, M Jake Pushie, Ewelina Stefaniak, and Kathryn L. Haas. “Structure and Affinity of Cu(I) Bound to Human Serum Albumin.” Inorganic Chemistry 56, no. 24 (December 2017): 15057–65. https://doi.org/10.1021/acs.inorgchem.7b02397.
- See also Schulte. et. al. listed above.
Cellular Cu uptake
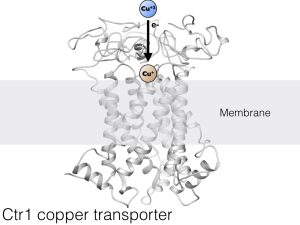
- Schwab, Stefanie, Jason Shearer, Steven E. Conklin, Bruno Alies, and Kathryn L. Haas. “Sequence proximity between Cu(II) and Cu(I) binding sites of human copper transporter 1 model peptides defines reactivity with ascorbate and O2.” Journal of Inorganic Biochemistry158 (May 2016): 70–76. https://doi.org/10.1016/j.jinorgbio.2015.12.021.
- Pushie, M Jake, Katharine Shaw, Katherine J. Franz, Jason Shearer, and Kathryn L. Haas. “Model Peptide Studies Reveal a Mixed Histidine-Methionine Cu(I) Binding Site at the N-Terminus of Human Copper Transporter 1.” Inorganic Chemistry 54, no. 17 (September 2015): 8544–51. https://doi.org/10.1021/acs.inorgchem.5b01162.