1. Long ncRNA-mediated gene expression, genome stability & cell fate decisions.
NcRNAs are noncoding transcripts that take part in epigenetic mechanisms by providing RNA-directed silencing, aiding recruitment of chromatin modifying complexes and in some instances, presenting enhancer-like functions to boost transcription (Fig 1). Altered expression of long ncRNAs has been implicated in various cancers, yet how long ncRNA-mediated pathways may impact tumorigenesis is still poorly understood.

Mammalian X-chromosome inactivation (XCI) provides a powerful model for studying the interplay between epigenetic mechanisms driven by long ncRNAs and development. This female specific dosage compensation mechanism depends on expression of Xist long ncRNA, accumulation of repressive chromatin modifications and DNA methylation, resulting in chromosome-wide silencing of the future Xi. While Xist expression is crucial for initiation of XCI, its role in maintenance of XCI is not clear. Interestingly, Xist deletions, increased X-linked gene dosage, and X-chromosome aneuploidy are often associated with variety of cancers such as breast, ovarian, and leukemias.
By deleting Xist specifically in blood cells of mice, we previously discovered that Xist plays an important role in hematopoietic stem cell (HSC) function and acts as a suppressor of cancer in mice (Yildirim et al., Cell 2013) suggesting a role for Xist in maintenance of XCI and a causal link between Xist loss and blood cancers.
Our laboratory uses primarily XCI as a model to understand how long ncRNAs:
- Regulate gene dosage and maintain epigenetic state,
- Impact genome stability,
- Participate in cell fate decisions.
Our long-term goal is to delineate the molecular bases and order of genetic, epigenetic and cellular processes that link altered expression of long ncRNAs and pathways driven by these molecules to disease and cancer.
2. Role of nuclear structure in gene expression regulation and genome function.
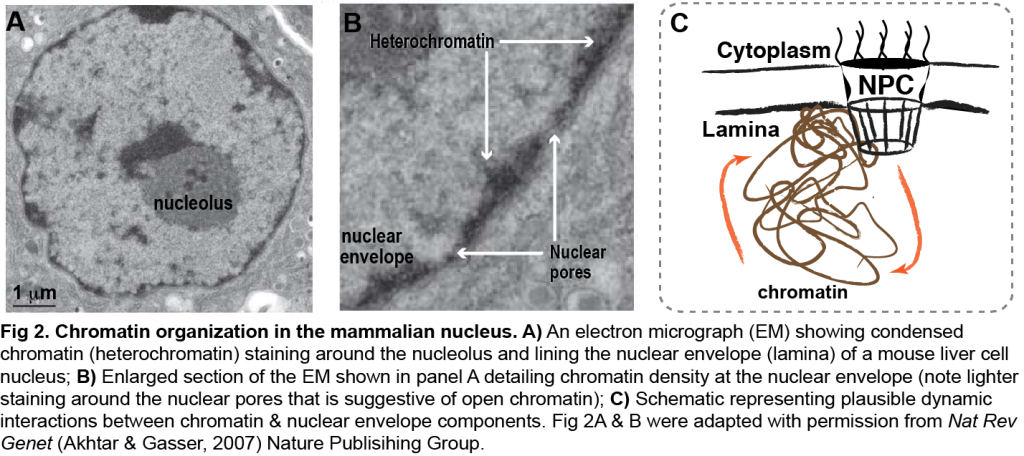
Components of the nuclear envelope (i.e. nuclear lamina, nuclear pore complex) maintain nuclear architecture and regulate nucleocytoplasmic trafficking (Fig 2). In addition to these canonical functions, data from various organisms provide evidence that components of the nuclear envelope interact with the genome and impact gene expression. Yet, the significance of these interactions in mammalian gene expression and genome function is poorly understood.
- Define the molecular bases of the interactions that are established between the genome and the components of the nuclear envelope,
- Determine how they regulate transcription and genome function.
Our findings will ultimately allow us to uncover how alterations in nuclear envelope components and chromatin configurations might induce uncontrolled cell divisions and migration patterns that lead to disease and cancer.