Arsenic in drinking water is a major health hazard to millions of people in South and East Asia and in other parts of the world. Long term arsenic exposure has been linked to cancer, heart disease, neuropathies and neurological sequelae, and to deficits in intelligence in children. Arsenic in water is normally ingested primarily as trivalent inorganic arsenic (iAs), which then undergoes hepatic methylation to methylarsonicacid (MMAs) and a second methylation to dimethylarsinic acid (DMAs). Each step involves a reduction from pentavalent to trivalent form. While the intermediate trivalent form of MMA is known to be highly toxic, the pentavalent form, DMAV, is more readily excreted in urine and facilitates elimination of As. This is evident in AS3MT deficient mice which demonstrate substantially higher As retention in tissues.
Since 2011, we have been engaged in a collaborative project with Mary Gamble and Megan Hall of Columbia University, professors of environmental health sciences and epidemiology, respectively. They conduct field studies in Bangladesh where a large portion of the population is subject to arsenic poisoning due to arsenic in the drinking water. One of their key goals is to identify inexpensive nutritional interventions that will help people to methylate arsenic and thereby facilitate arsenic elimination in urine. The methylation reactions in the liver that detoxify arsenic occur in the methionine cycle and are influenced by the folate cycle, parts of cell metabolism for which we have created mathematical models, so they and we were natural collaborators.
The Folate and Methionine Cycles (click figure)
S-andenosyl-methionine(SAM), a metabolite of methionine, is the universal methyl group donor is cells. The SAM concentration is influenced by the folate cycle and the rest of one-carbon metabolism via the methionine synthase(MS) reaction that remethylates homocysteine to methionine. It is known both from experimentation and from modeling that an increase in folate status increases the concentration of SAM in hepatic cells. Thus one might predict that increasing folate status in individuals would increase the rate of methylation of iAs and help to detoxify arsenic faster. Gamble and collaborators verified that this is true in field trials in Bangladesh in 2006 and 2007 (for complete references, see Lawley et al. (2011)). So, our first collaborative project was to create a whole body model of folate intake, storage, detoxification in the liver, and excretion in the urine. Sean Lawley, then a mathematics graduate student at Duke, played a key role in the project and was first author on the paper (Lawley et al.(2011)).
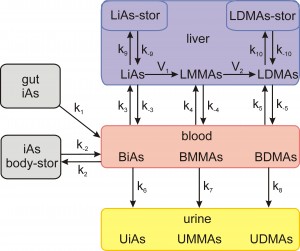
Whole Body Arsenic Metabolism (click figure)
We tested the whole body model by showing that, with no changes in parameters, it predicts accurately the time courses of urinary excretion in multiple dose experiments conducted on human subjects. But, our main purpose was to use the model to study and interpret the data on the effects of folate supplementation on arsenic methylation and excretion in clinical trials in Bangladesh. Folate supplementation of folate-deficient individuals resulted in a 14% decrease in arsenicals in the blood. This was confirmed by the model and the model also predicted that arsenicals in the liver will decrease by 19% and arsenicals in other body stores by 26% in these same individuals. In addition, the model predicts that arsenic methyltransferase has been upregulated by a factor of two in the Bangladesh population. Finally, we also showed that a modification of the model gives excellent fits to the data on arsenic metabolism in human cultured hepatocytes.
Our second project was to study the effect of glutathione(GSH) on arsenic methylation. GSH appears to play three distinct roles. First, GSH is the main anti-oxidant in cells, and it is used in the reduction step from MMAsv to MMAsIII and the reduction step from DMAsv to DMAsIII. Secondly, GSH can conjugate iAs, MMAs, and DMAs. Finally, GSH appears to bind to arsenic methyltransferase (AS3MT) and to increase its activity. In a complicated physiological and biochemical situation such as this, mathematical modeling can be a useful tool for sorting out the consequences of different hypotheses and for helping to interpret experimental data. So we created mathematical model of the arsenic methylation pathway which incorporates three different roles for GSH. From a biochemical point of view the pathway is simple but highly interesting. Both iAs and MMAs show substrate inhibition for AS3MT. MMAsv shows both product inhibition and substrate inhibition for AS3MT. Sean Lawley, then a graduate student at Duke, was a key player in this project and was first author on the paper (Lawley et al. (2014)).
Glutathione and Arsenic Methylation (click figure)
We showed that the model predicts and helps explain recent experimental data on the effects of glutathione on arsenic methylation. We explain why the experimental data imply that MMAs acid inhibits the second methylation step. The accurately model predicted time course data from recent experimental studies. We explained why increasing glutathione when it is low increases arsenic methylation and that at very high concentrations increasing glutathione decreases methylation. We explain why the possible temporal variation of the glutathione concentration affects the interpretation of experimental studies that last hours.
One of the most interesting ideas of our collaborators is that feeding individuals creatine could increase the arsenic methylation rate. Creatine is produced by a two step process. In the first step the enzyme L-arginine:glycine amidinotransferase (AGAT) makes guanadino acetate (GAA) in the kidney from arginine and glycine. GAA is exported into the plasma and is taken up by the liver where it receives a methyl group from SAM and becomes creatine via the GAMT reaction (see the figure below). The creatine is exported from the liver and used muscle tissue. Normally mammals ingest about 50 % of the creatine they need and synthesize the other half through these reactions. Thus, one would expect that creatine synthesis in the liver would be sensitive to the amount of creatine in the diet, and indeed it is. High plasma concentrations in the plasma inhibit AGAT in the kidney so that less GAA is sent to the liver and thus less creatine is produced by the GAMT reaction. Since the GAMT reaction is running more slowly, SAM should rise and thus more methyl groups will be available for arsenic detoxification (AS3MT).
Regulation of Competing Methyltransferases (click figure)
This led us to consider the following general question. There are about 150 methyltransferase (MT) reactions running in parallel in cells (only 5 are pictured in the figure). They all use SAM as a substrate and they all produce S-adenosyl-homocysteine(SAH) as a product and this presents the cell with an important regulatory problem. If the flux along one pathway is changed then the SAM concentration will change affecting all the other MT pathways, so it is difficult for the cell to regulate the pathways independently. The flux along each pathway is dependent on enzyme activity, SAM concentration, co-substrate concentration, and in some cases allosteric inhibition. But how does the cell overcome the challenge that the flux on each pathway affects the flux on all the others? That is, how does it maintain homeostasis on the other pathways? It is not surprising that there are many different regulatory mechanisms that the cell uses to met this challenge. These regulatory mechanisms are studied in (Reed et al. (2015)).
About 30 years ago, Conrad Wagner and co-workers discovered that 5mTHF in the folate cycle binds to GNMT and lowers its activity. They refer to GNMT as the “salvage pathway.’’ If SAM is high, then MTHFR is inhibited, so 5mTHF concentration declines. This removes inhibition of GNMT so more methyl groups are sent down the GNMT pathway, which keeps SAM from rising too much. On the other hand, if SAM is low, then the inhibition of MTHFR is withdrawn and 5mTHF rises inhibiting GNMT more, so fewer methyl groups go on the GNMT pathway, saving them for the other MT pathways. In the past decade Wagner and Luka have provided much more information about the binding of 5mTHF to GNMT. In particular, each molecule of GNMT can bind two molecules of 5mTHF (see the figure above) and when both sites are occupied, GNMT loses its activity. In (clickable reference) we give computational evidence that once bound GNMT retains half of its activity.
Now we return to the question of creatine supplementation for arsenic detoxification. If one supplements the diet with creatine then one expects the flux on the GAMT pathway will drop dramatically and SAM will rise making more methyl groups available for AS3MT. But this is exactly the scenario that the regulations referred to above help the cell to avoid (the changed flux on one pathway affecting all the others). So for the creatine hypothesis to work well, one needs to break some of the regulatory homeostatic mechanisms. And, folate supplementation does just that. Not only does folate supplementation raise the SAM concentration, but it increases the concentration of 5mTHF. This lowers the activity of GNMT reducing the effectiveness of the “salvage pathway” that is one of the homeostatic mechanisms. Here are two recent references on creatine supplementation:
Peters et al. 2015. Low-Dose Creatine Supplementation Lowers Plasma Guanidinoacetate, but Not Plasma Homocysteine, in a Double-Blind, Randomized, Placebo-Controlled Trial. J. Nutr. 145:2245-52;
Peters et al. 2015. Folic acid and creatine as therapeutic approaches to lower blood arsenic: A Randomized-Controlled Trial. Envir. Health Persp. 123:1294-1301.)
This is a perfect illustration of why mathematical models are an essential tool for the investigation of public health interventions. If one understands how the underlying biochemical mechanisms work, then one can design wise intervention strategies.