Parkinson’s disease has been traditionally thought of as a dopaminergic disease in whichcells of the substantia nigra pars compacta (SNc) die. However, accumulating evidence implies an important role for the serotonergic system in Parkinson’s disease in general and in physiological responses to levodopa therapy, the first line of treatment. We used a mathematical model (Reed et al. 2012) to investigate the consequences of levodopa therapy on the serotonergic system and on the pulsatile release of dopamine (DA) from dopaminergic and serotonergic terminals in the striatum.
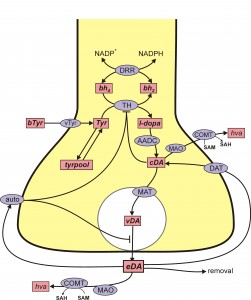
There are two key ideas that are necessary for understanding the complicated and important connections between the serotonin (5HT) system and the dopamine (DA) system. The first idea is the recognition of the similarities in the synthesis pathways of 5HT and DA in 5HT and DA terminals. The second is volume transmission, which is discussed in a different tab and further below. The following figures show schematically the synthesis, vesicular packaging, release into the extracellular space, reuptake, and control by autoreceptors of DA and 5HT.
DA is synthesized from the amino acid tyrosine (try) that crosses the blood-brain barrier and is taken up by DA nerve terminals by the L-transporter. In the DA terminal, the enzyme tyrosine hydroxylase (TH) adds an OH group to tyr making levodopa (l-dopa). We will abbreviate levodopa by l-dopa and by LD. The amino acid decarboxylase (AADC) cuts off the carboxyl group to make DA, which is indicated by cda, cytosolic DA. The monoamine transporter (MAT) packages cda into vesicles making vda. When the action potential arrives a sequence of events, including Ca++ influx, causes some vesicles to move to the boundary of the terminal and release their contents into the extracellular space. The extracellular DA, e da, is taken back up into the cytosol by the dopamine transporter (DAT). Extracellular DA also binds to DA autoreceptors (auto) that inhibit synthesis and release. This is clearly a control mechanism intended to stabilize the concentration of eda. Of course, the actual situation is more complicated, for example, cda itself inhibits TH and eda can be taken up by glial cells.
da, is taken back up into the cytosol by the dopamine transporter (DAT). Extracellular DA also binds to DA autoreceptors (auto) that inhibit synthesis and release. This is clearly a control mechanism intended to stabilize the concentration of eda. Of course, the actual situation is more complicated, for example, cda itself inhibits TH and eda can be taken up by glial cells.
The situation for 5HT is remarkably similar. 5HT is synthesized from the amino acid tryptophan (tryp) that crosses the blood-brain barrier and is taken up by 5HT nerve terminals by the L-transporter. In the 5HT terminal, the enzyme tryptophan hydroxylase (TPH) adds an OH group to tryp making 5-htp. The enzyme amino acid decarboxylase (AADC) cuts off the carboxyl group to make 5HT, which is indicated by c5ht, cytosolic 5HT. The monoamine transporter (MAT) packages c5ht into vesicles making v5ht. When the action potential arrives a sequence of events, including Ca++ influx, causes some vesicles to move to the boundary of the terminal and release their contents into the extracellular space. The extracellular 5HT, e5ht, is taken back up into the cytosol by the serotonin transporter (SERT). Extracellular 5HT also binds to 5HT autoreceptors (auto) that inhibit synthesis and release.
All cells in the body take up tyrosine and tryptophan. The only difference between DA neurons and 5HT neurons is that DA neurons express the enzyme TH and thus make DA and 5HT neurons express TPH and thus make 5HT. As we will explain, this distinction is eliminated in 5HT neurons when one gives a dose of levodopa.
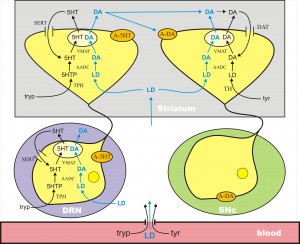
In Parkinson’s disease, the DA cells in the substantia nigra pars compacta (SNc) die. These cells project to the striatum where low levels of DA cause dysfunction in the motor system. DA does not cross the blood-brain barrier because it doesn’t have a carboxyl group and is not recognized as an amino acid. However, its precursor, l-dopa, still has the carboxyl group and does cross the blood-brain barrier. Thus, the idea of levodopa therapy is to fill the remaining DA terminals in the striatum with l-dopa so that these remaining terminals will release more DA into the extracellular space when action potentials arrive, compensating for the DA terminal loss caused by cell death in the SNc.
L-dopa is taken up into all cells by the L-transporter, just like tyr and tryp. When l-dopa is taken up into 5HT terminals the enzyme AADC cuts off the carboxyl group to make cda, which is then packaged into vesicles by MAT. Thus vesicles in the 5HT neurons are filled with both 5HT and DA, and when the action potential arrives both are released into the extracellular space. There is a large dense projection of 5HT neurons in the dorsal raphe nucleus to the striatum. So, during a dose of levodopa, the 5HT neurons release a large pulse of DA into the striatum.
All the aspects of this story have been verified experimentally in the last 10 years. Experiments have verified that 5HT neurons can store and release DA in vivo and in vitro. In levodopa treatment of a hemiparkinsonian rat, striatal extracellular DA decreased substantially when the serotonergic system was lesioned. Glial cells also express AADC and so could contribute to the conversion of LD to DA, but experiments using reserpine to block vesicular packaging showed a great reduction of extracellular DA, suggesting that most of the levodopa-derived DA is released by exocytosis of vesicles rather than by glia, at least at physiological levels of levodopa administration. It has also been shown that 5HT1a autoreceptor agonists (that decrease RN firing) and 5HT1b autoreceptor agonists (that decrease release at 5HT terminals) both lower extracellular DA in the striatum in a dose-dependent manner after an LD dose.
The new understanding of 5HT neurons in levodopa therapy has helped to explain a serious side effects of levodopa therapy. Within 5 years of chronic LD treatment, many patients experience a variety of complications (Mouradian et al., 1988). For instance, the length of the therapeutic time window in which a given LD dose relieves PD symptoms gradually shortens and approaches the plasma half-life of LD (wearing-off). Rapid variations in efficacy may occur (on-off fluctuations). Another, particularly troubling, complication of chronic LD therapy is the appearance of involuntary movements (levodopa-induced dyskinesia, LID). These complications increase patients’ disability substantially, pose a therapeutic dilemma, and limit the use of LD.
There is good evidence that large pulses of DA in the striatum are the proximal cause of LID that are seen in long-term dosing. And there is conclusive evidence that these large pulses result from DA release from 5HT neurons in the striatum. Lesioning the 5HT system or giving selective serotonin autoreceptor (5HT1a and 5HT1b) agonists results in a nearly complete elimination of LID.
In order to investigate these phenomena, we created a mathematical model (Reed et al., 2012) that corresponds to the above diagram. What we discovered was that the size of these large pulses of DA coming from 5HT neurons depended critically on the fraction, f, of SNc cells left alive, which is why there are more and more dyskinesias and Parkinson’s disease progresses. Here is the intuitive explanation. As long as there are lots of SNc cells alive, there will be lots of DA terminals in the striatum with DAT and DA autoreceptors. The DATs take up a lot of the excess DA that comes from the 5HT neurons and the DA autoreceptors restrict DA release from the DA terminals when the extracellular DA concentration is high. However, as the fraction of SNc cells left alive gets smaller and smaller these two control mechanisms have less and less effect. The DA from 5HT neurons is released much faster causing high pulses of DA in the striatum and dyskinesias, and in addition the extra DA created by the levodopa dose is used up much faster shortening the period of efficacy of the LD dose.
Legend. (A) Shows the time course of extracellular DA in the striatum for different values of f, the fraction of SNc cells left alive. As f declines the curves get higher because there are fewer DATs to take up the DA released by 5HT neurons. However, when f is very small the peaks decline because removal mechanisms such as catabolism, diffusion, and uptake into glial cells become more important. The dashed black horizontal line in (A) represents the level of extracellular DA needed in the striatum for anti-Parkinsonian effects. (B) Reproduces the first three curves from (A) (solid curves, f = 1, f = 0.2, f = 0.1). The dashed curves in the same color show the amount of extracellular DA in the striatum that comes from the DA neurons. For a normal individual (f = 1) the DA neurons contribute approximately 60%, but as SNc cells die (f = 0.2, f = 0.1) most of the DA comes from the 5HT neurons. (C) Shows that the amount of time that extracellular DA stays above the therapeutic level (the dashed black line in (A)) declines as PD progresses until it becomes approximately 2 h.
We remark that it has been shown in animals that during an LD dose the 5HT released in projection regions is about half of normal. It is interesting to speculate whether this might be a partial cause of depression in some Parkinson’s patients.