Once released into the air, the vapor can be breathed into the lungs, where it rapidly gains access to the bloodstream. The lungs have a very large surface area for absorption of drugs1, toxins and other compounds from the alveoli (very small sacs where gas is exchanged) into the capillaries. Capillaries are the smallest form of blood vessels and are very numerous. In fact they are able to deliver nutrients such as oxygen and glucose to every cell in the body. They also pick up waste such as carbon dioxide and metabolic products. Capillaries (made up of endothelial cells2) have numerous pores (“fenestra”3 – latin for windows). These pores are actually spaces between the endothelial cells and they are larger than the small pores found in other kinds of cell membranes. The fenestrae allow large molecules (up to molecular weights of 25,000 daltons) and charged molecules to pass through without difficulty (Figure 2). So capillaries are much less restrictive to the passage of solutes. This property allows large molecules such as proteins and water-soluble vitamins to be delivered to other cells throughout the body. In the case of nerve gas, the small size and lipophilic4 (lipid-loving) nature of the molecules allows them to pass through alveolar cell membranes (which are much like the capillary membranes) into the capillaries without any difficulty. In the capillaries, the nerve gas is dissolved in the blood (i.e. it is no longer in a gaseous form) because there is so little of it and it has high solubility in aqueous environments. The nerve gas travels in the oxygenated blood to the heart and then gets pumped throughout the body (organs such as brain, liver and kidneys that have a high blood flow receive blood first) to reach all cells.
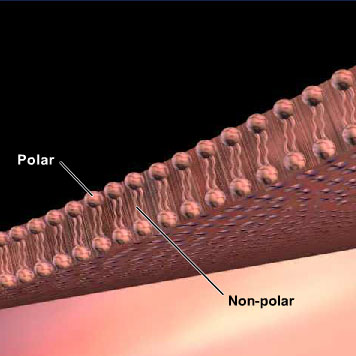
Nerve gas5 can also be absorbed through the skin (epithelial) cells into the capillaries. It is fairly difficult for most drugs to gain access to the bloodstream from the skin because there are several layers of skin and the blood supply to the outermost layers is very sparse. However, in the case of nerve gas, these compounds are very lipophilic and can penetrate through the layers of skin cells easily. The cell membrane is a sandwich (bilayer) of lipids, with the polar6 or hydrophilic7 (water-loving) headgroups arranged at the surfaces of the membrane and the non-polar8 or hydrophobic9 (water-fearing) fatty acid carbon chains in the middle (see Figure 3). Drugs that are hydrophobic (lipophilic or non-polar) or uncharged penetrate epithelial cell membranes easily because they dissolve in the hydrophobic core of the membrane. Hydrophilic (ionized10 or polar) compounds cannot penetrate the lipid interior of the membrane and they remain along the hydrophilic portions of the membrane. The lipophilic molecules (like nerve gas) are able to pass through the membrane along their concentration gradient (passive diffusion11) from the side of higher concentration to the side of lower concentration, until an equilibrium is reached.
Nerve gas penetrates the eyes as well. It can diffuse easily through the cornea, the schlera (white) and the conjunctiva (epithelial tissue near the corner). In addition, the gas can enter the capillaries embedded in the schlera. Thus, nerve gas has several effects on the eyes.
Regardless of the method of exposure, nerve gas gets into the brain very easily. This is also due to its lipophilic nature. The brain permits the entry of certain kinds of drugs or poisons; only those compounds that are highly lipophilic (i.e. uncharged or unionized) are able to penetrate the group of membranes that form the “blood brain barrier”12. The blood brain barrier consists of tightly packed capillary endothelial cells, so there are no pores through which charged compounds can pass (Figure 2). Compounds that are highly lipophilic such as nerve gas penetrate most quickly. By restricting only certain molecules (and drugs) from reaching the brain, the brain can be protected from many (but not all!) dangerous compounds.
Definitions:
1 a substance that affects the structure or function of a cell or organism.
2 cells that line the blood vessels and capillaries. Unlike epithelial cells, these cells have no contact with the environment outside the body.
3 small spaces or pores within endothelial cells that form the capillary membrane. These pores allow charged drugs or larger drugs to pass through the capillaries.
4 high lipid solubility. Lipophilic compounds dissolve readily in oil or organic solvent. They exist in an uncharged or non-polar form and cross biological membranes very easily.
5 a group of very lipophilic compounds (e.g. sarin, tabun, soman) that can exist as a vapor at room temperature. They contain phosphorus groups and bind avidly to acetylcholinesterase to inhibit its activity. The inhibition of acetylcholinesterase causes the accumulation of acetylcholine in all areas of the nervous system, causing excessive muscle contraction followed by paralysis, secretions, seizures and death by respiratory failure.
6 a chemical property of a substance that indicates an uneven distribution of charge within the molecule. A polar substance or drug mixes well with water but not with organic solvents and lipids. Polar or charged compounds do not cross cell membranes (lipid) very easily.
7 dissolves readily in water. Hydrophilic compounds exist in an ionized or polar form and have difficulty crossing biological membranes (except capillary membranes).
8 a chemical property of a substance that indicates an even distribution of charge within the molecule. A non-polar or non-charged compound mixes well with organic solvents and lipids but not with water.
9 “water-fearing”; a compound that is soluble in fat but not water. This is typical of compounds with chains of C atoms.
10 an atom, radical, or molecule that has gained or lost one or more electrons. Therefore it acquires a net negative or positive charge.
11 the movement of a solute in its uncharged form to cross a membrane along a concentration gradient. No energy is required.
12 a tightly joined layer of cells lining the capillaries in the brain. It restricts passage of drugs and other molecules across the cell layer into the brain to include only those that are lipophilic (uncharged).
Figures:
Figure 2 Cross section of capillary showing endothelial cells. In the non-brain capillary fenestrae are present. In brain capillaries the endothelial cells are tightly packed and no fenestrae are present.
Figure 3 Schematic view of a cell membrane. Lipids are arranged with polar head-groups facing the outside and inside of the cell, while the fatty acid chains form the non-polar (hydrophobic) membrane interior.