Adipose tissues and the pathophysiology of obesity
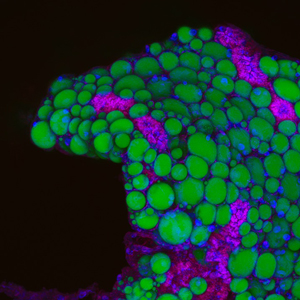 Fat stored in white adipose tissues (AT) provides critical energy reserves during periods of undernutrition, and undergoes pathologic expansion and inflammation during obesity. It is known that manipulation of intestinal microbiome can affect AT expansion, metabolism, and inflammation, but underlying mechanisms remain unresolved. We have established the zebrafish a model for AT and obesity research. We established methods for visualizing zebrafish AT in vivo, and uncovered extensive homologies between zebrafish and mammalian AT. We also developed the first anatomic atlas of zebrafish AT and a quantitative phenotyping technique for linear discriminant analysis of zebrafish adiposity traits. Demonstrating the utility of this model system, we identified PLXND1 as a conserved regulator of body fat distribution and insulin sensitivity in humans and zebrafish. Current work in our lab is using the zebrafish model system to identify environmental factors that regulate adiposity and to understand the underlying molecular mechanisms.
Fat stored in white adipose tissues (AT) provides critical energy reserves during periods of undernutrition, and undergoes pathologic expansion and inflammation during obesity. It is known that manipulation of intestinal microbiome can affect AT expansion, metabolism, and inflammation, but underlying mechanisms remain unresolved. We have established the zebrafish a model for AT and obesity research. We established methods for visualizing zebrafish AT in vivo, and uncovered extensive homologies between zebrafish and mammalian AT. We also developed the first anatomic atlas of zebrafish AT and a quantitative phenotyping technique for linear discriminant analysis of zebrafish adiposity traits. Demonstrating the utility of this model system, we identified PLXND1 as a conserved regulator of body fat distribution and insulin sensitivity in humans and zebrafish. Current work in our lab is using the zebrafish model system to identify environmental factors that regulate adiposity and to understand the underlying molecular mechanisms.
Human observational studies and fecal transplantation studies in animal models (both largely focused on adults) have identified interconnections among obesity, insulin resistance, and the gut microbiome. Because epidemiology points to childhood origins for the genesis of obesity, there is a critical need to understand the mechanisms of pediatric obesity and to develop tools for its prediction, prevention, and treatment. Children with severe obesity mimic adult phenotypes in their development of metabolic and cardiovascular risk, and yet are at the earliest stages of disease with fewer and less severe co-morbid conditions. Children with obesity therefore presented an ideal population in which to garner deeper insights into the obesity-associated microbiome. To enable such insights, we have established a comprehensive research resource at Duke to define mechanisms underlying microbial regulation of host metabolism in adolescents with obesity before and after weight loss intervention. We are using this resource, called the Pediatric Obesity Microbiome and Metabolism Study (POMMS), to define associations between intestinal microbiome and obesity and weight loss intervention. In parallel, we are using animal models and bacterial genetic analysis to identify the molecular mechanisms by which human intestinal bacteria regulate metabolic traits linked to pediatric obesity.
Relevant publications:
Flynn, E.J., Trent, C.M., and Rawls, J.F. (2009) Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J. Lipid Res. 50(8): 1641-1652.
[PMCID: PMC2724053]
Minchin, J.E.N., Dahlman, I., Harvey, C.J., Mejhert, N., Singh, M.K., Epstein, J.A., Arner, P., Torres-Vázquez, J., and Rawls, J.F. (2015) Plexin D1 determines body fat distribution by regulating the type V collagen microenvironment in visceral adipose tissue. Proc Natl Acad Sci USA 112(14): 4363-8. [PMCID: PMC4394244]
Minchin, J.E.N, and Rawls, J.F. (2017) In vivo imaging and quantification of regional adiposity in zebrafish. Methods in Cell Biology. 138: 3-27. [PMCID: PMC5497849]
Minchin, J.E.N., and Rawls, J.F. (2017) A classification system for zebrafish adipose tissues. Dis. Models & Mech. 10: 797-809. [PMCID: PMC5482999]
Minchin, J.E.N., Scahill, C.M., Staudt, N., Busch-Nentwich, E.M., & Rawls J.F. (2018) Deep phenotyping reveals genetic and diet-induced adiposity changes in zebrafish. J. Lipid Res. 59(8):1536-1545. [PMCID: PMC6071777]