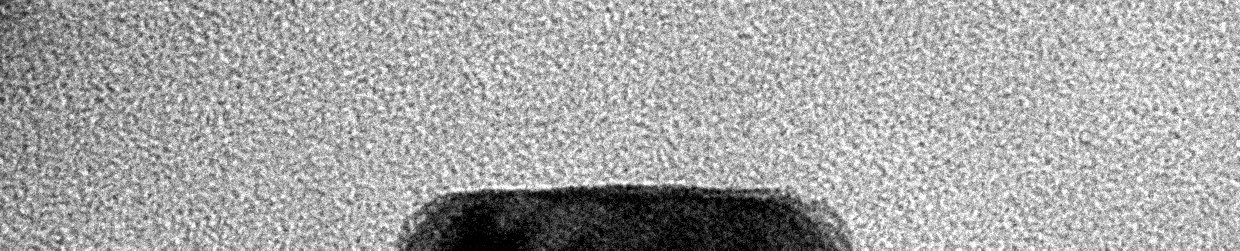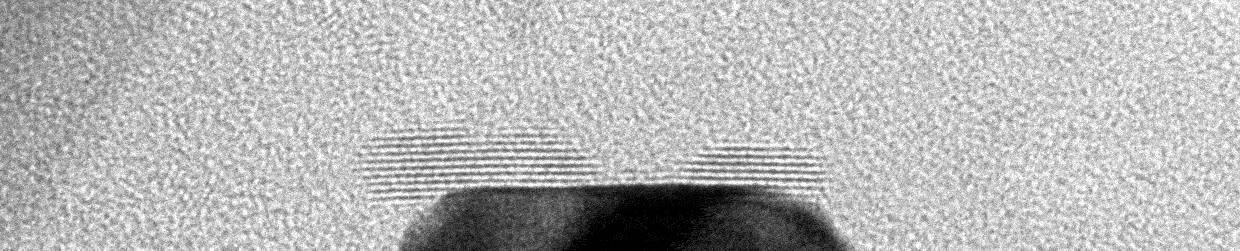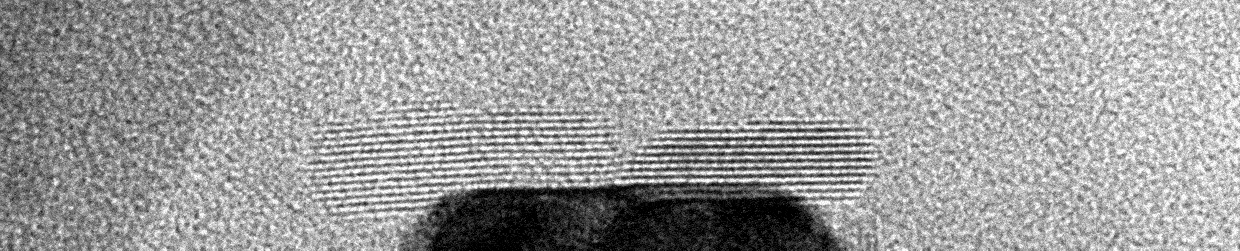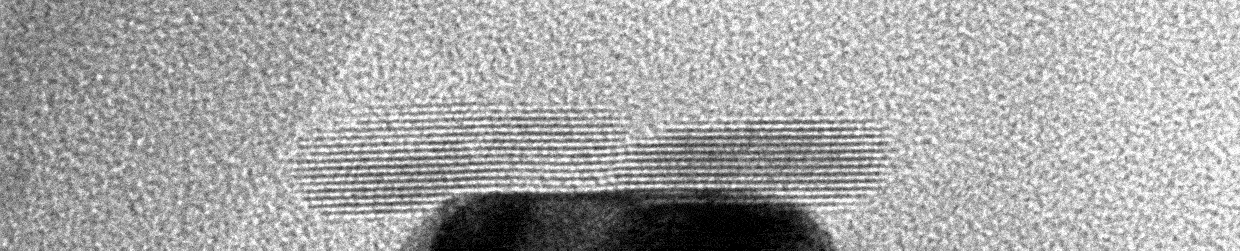
Liquid Phase Transmission Electron Microscopy
Matter can undergo changes in structure and composition in liquid environments, including in electrocatalysts used for electrochemical conversions, batteries charging and discharging, and materials corroding in harsh environments. These changes can be readily observed on a human scale as degradation of material performance or the activation of materials over time. However, little is known about how these changes occur on the atomic scale. The Moreno-Hernandez Laboratory specializes in the study of the structural dynamics of nanomaterials with liquid phase transmission electron microscopy, a technique that allows the observation of atomic changes in structure in liquid environments in real time. These studies have revealed previously unknown mechanisms of dissolution of nanomaterials and ever-present heterogeneity in nanoscale properties. Information from these studies is being utilized to design next-generation nanomaterials with exceptional properties and guide the development of spatiotemporal theories of matter.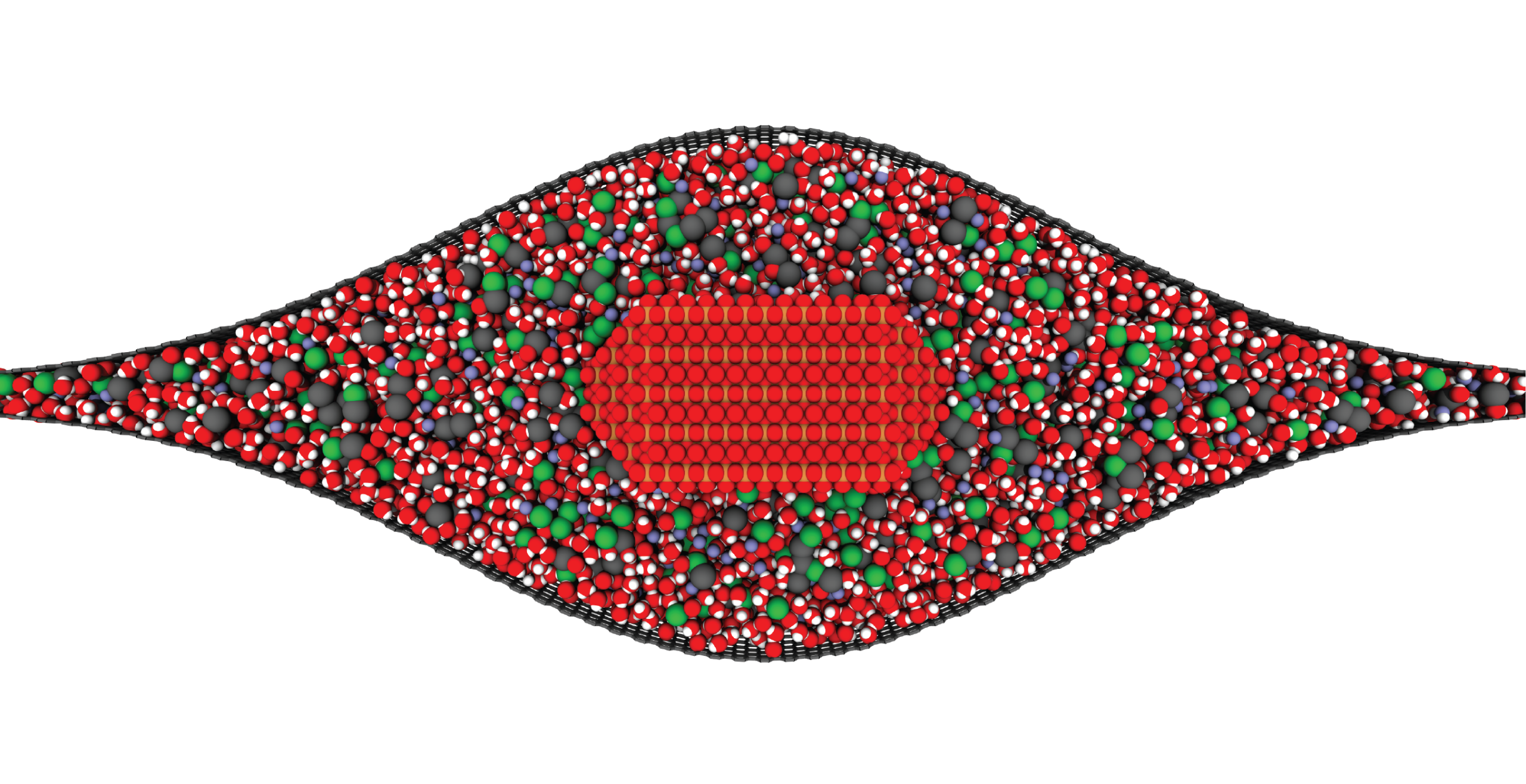 Nanomaterial Design
Nanomaterial Design
Materials have the potential to enable next-generation energy conversion and quantum information technologies. In recent years, advances in manufacturing processes have enhanced control of material growth at the nanoscale, enabling the synthesis of complex nanomaterials consisting of many elements via fine control of the growth conditions. However, the synthesis of nanomaterials with desired properties remains a challenge for a wide variety of applications. In the Moreno-Hernandez Laboratory we specialize in the synthesis of precisely defined nanocrystals of metal oxides that enable fundamental studies that elucidate the influence of material chemistry and structure on material properties such as nanoscale structural dynamics and energy conversion performance. These studies are leveraged to carefully guide the design of next-generation nanomaterials in complex compositional spaces with properties that surpass state-of-the art materials. Electrochemical Energy Conversions
Electrochemical Energy Conversions
Electrocatalysts are an important component of a future chemical infrastructure, allowing electrons to serve as reagents in the synthesis of important chemicals with applications in renewable energy storage, water sanitation, pharmaceuticals, and polymers. Additionally, energy storage via batteries has become an integral part of our society’s energy infrastructure, enabling technologies such as wireless personal computing devices. These transformations are powered by materials and nanomaterials that enable electrical energy to be directly coupled to chemical reactions. However, further advances in material chemistry are needed to improve the stability and efficiency of electrochemical devices. In the Moreno-Hernandez laboratory we specialize in the development of devices that enable electrochemical energy conversions, such as electrolyzers, that are powered by next-generation nanomaterials. Assessment of device stability and efficiency at relevant operating conditions provides key benchmarks of material performance, which accelerate the development of materials with potential to meet the growing demand of electrochemical technologies.