One-Step High Entropy Oxide Synthesis
High entropy catalysts are materials consisting of 5 or more elements in concentrations of 5-35 at% with uniform distribution. High entropy oxides have shown to be good catalyst supports for many chemical reactions. However, the preparation of metal nanoparticle catalysts supported on metal oxide support is time-consuming and requires multiple complicated steps. Herein, we use a one-step glycine-nitrate combustion method to synthesize highly dispersed rhodium species on a high surface area high entropy oxide. This catalyst shows a high selectivity to produce CO in CO2 hydrogenation with much greater activity compared to other rhodium-based catalysts. We’ve also studied the effect of different metal elements in HEO and demonstrated that high CO selectivity is achieved if one of the metals in the metal oxide support favors CO production. We identified that copper and zinc are responsible for high CO selectivity due to their low *CO binding strength. During hydrogenation, a strong metal-support interaction (SMSI) was created through charge transfer and formed an encapsulated structure between rhodium species and HEO support to lower the *CO binding strength, which enables high CO selectivity in the reaction.
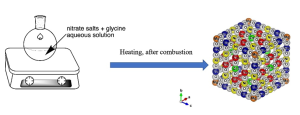
Scheme 1: One step synthesis of high entropy oxide (MgCoNiCuZn)O

Figure A: SEM image of high entropy oxide (MgCoNiCuZn)O
