Personalized Glioblastoma Drug Development
We will develop therapies based on the molecular features of each patient’s tumor. Each patient’s tumor will be subjected to detailed proteomic and genomic analyses to identify the molecular machinery driving its growth. From this information, we will identify a list of drugs that have the potential to block the specific growth-promoting molecular machinery. These drugs will be tested in preclinical models (xenografts and stem cells) developed from the patient’s tumor, as these models retain the molecular machinery of the original tumor. Drugs found to be effective in the patient’s-derived preclinical models will be given to the patient.
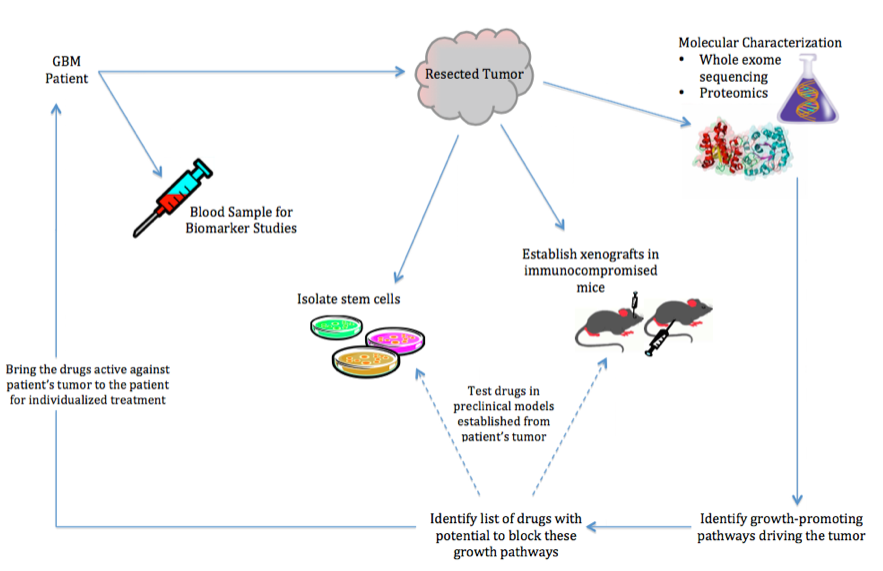
Ongoing GBM Drug Development Projects
- Brain-penetrating irreversible EGFR tyrosine kinase inhibitor for the subset of GBMs with activated EGFR—funded by the NIH/NCATS
- Evaluation of AZD9291 in Glioblastoma Patients with Activated EGFR – Glioblastoma (GBM) is one of the most aggressive and common brain tumors in adults. Current treatments have failed to extend median survival time beyond fifteen months, indicating an urgent need for a more effective GBM therapy. To achieve this goal, scientists must better understand the molecular machinery that drives GBM. Recent work indicates that a subset of GBM tumors show elevated activity of a protein known to affect tumor growth called epidermal growth factor receptor (EGFR). Tumors with overactive EGFR also have enhanced activity of another protein called HER2. AZD9291 — a compound that can cross the blood-brain barrier and was originally designed to treat lung cancer — blocks the activity of both EGFR and HER2. The proposed studies are designed to find an effective therapy for glioblastoma patients with an activated form of EGFR. This work could lead to the development of a new, more effective and more precise treatment strategy for a specific group of GBM patients.
- You can read our press release on Duke Translational Medicine Institute’s website here.
- Development of an FDA-approved NK1R blocker for the subset of GBMs with activated NK1R—funded by Helsinn.
