The Duke/UNC ADRC curates resources from active protocols, with new data and samples being logged every day. Additionally, investigators in our Center have decades of experience in dementia-related studies that continue to be a rich resource for further research. Descriptions and contact information for each cohort and resource are provided below.
Active protocols
The following resources are derived from active protocols, with ongoing enrollment and data collection:

Duke-UNC Memory & Aging Study (MAS) and Young Cohort
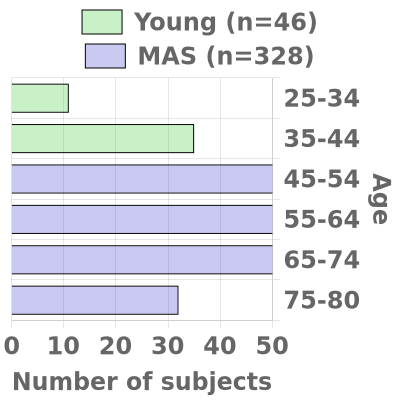
The Duke/UNC ADRC oversees two cohorts, the Memory & Aging Study (MAS) (n=420 over five years) and the Young Cohort (n=120). Participants from both cohorts are recruited from the Triangle and Eastern North Carolina.
See what data is available: ARENA Gateway: ADRC cohorts
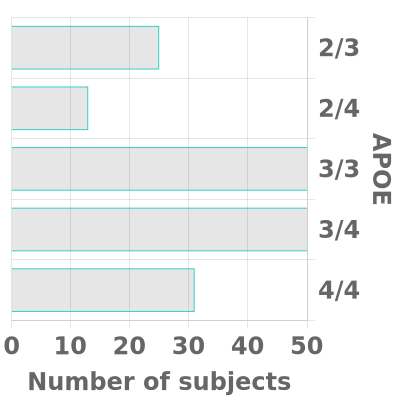
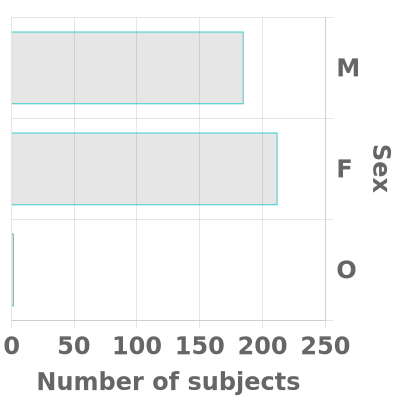
We strive to have substantial rural (~30%) and African-American or other Non-White (~20%) enrollment in both cohorts and our goal is for each cohort to be fairly evenly balanced between men and women. In each cohort, we aim for approximately two-thirds of participants to have a known APOE4 gene and/or a family history of Alzheimer’s disease in a first-degree relative (or in a grandparent, in the case of the Young Cohort).
Cohorts
- Memory and Aging Study (MAS) Cohort (Ages 45 to 80)
MAS will develop and maintain a prospectively followed cohort of 420 research participants, ages 45 to 80 and this will enable us to observe key transition periods related to AD risk, including menopause, onset of comorbidities, and the transition to MCI and dementia. To accomplish these goals, the cohort design will collect from two different groups based on affection. The first group will consist of 320 participants who are cognitively normal at entry and roughly equal in age distribution. The second group will include 100 participants symptomatic at entry (MCI or early dementia).
- Young Cohort (Ages 25 to 44)
The Young Cohort (n=120) will consist of participants ages 25-44 who will undergo a one-time evaluation and biomarker collection. The availability of this unique comparison group will enable investigators to evaluate the degree to which novel biomarkers or signatures associated with AD in older cohorts are linked to other contributing factors, such as age or genetic risk.
- Each participant contributes UDS-based medical, neurologic, psychiatric, and neuropsychological evaluations.
- We also collect blood, urine, and cerebrospinal fluid specimens.
- During the baseline visit, we collect motor-sensory assessments, and imaging:
- brain MRI (T1, T2 FLAIR, diffusion-weighted, fMRI resting-state, susceptibility)
- retinal imaging
Corrected distance visual acuity on Snellen chartZeiss Cirrus HD-5000 AngioPlex retinal and optic nerve imaging device
- Optical coherence tomography (OCT)
- macular cube
- measures ganglion cell inner plexiform layer thickness, central subfield thickness
- HD21 raster scan
- measures subfoveal choroidal thickness
- optic disc cube
- measures retinal nerve fiber layer thickness
- macular cube
- Optical coherence tomography angiography (OCTA)
- 3x3mm OCTA scan centered on macula
- images the superficial capillary plexus of the retina
- measures foveal avascular zone area, vessel density, and perfusion density
- 6x6mm OCTA scan centered on macula
- images the superficial capillary plexus of the retina
- measures vessel density and perfusion density
- 4.5x4.5mm OCTA scan centered on optic disc
- images the superficial capillary plexus of the retina
- measures capillary perfusion density and capillary flux index
- 3x3mm OCTA scan centered on macula
Optos California Scanning Laser Ophthalmoscopy Camera- Ultrawidefield color fundus photographs (~220 degrees)
- can measure vessel width gradient, vessel tortuosity, fractal dimension
- Ultrawidefield fundus autofluorescence (~220 degrees)
- Optical coherence tomography (OCT)
- Up to 100 participants in the MAS cohort who are unable or unwilling to undergo lumbar puncture may be consented to undergo a PET image of their brain, rather than contributing cerebrospinal fluid via lumbar puncture.
- The participant’s amyloid and tau status is determined either from cerebrospinal fluid or a PET image.
- Participants who are peri-menopausal women also contribute a set of questionnaires about menopause symptoms and extra blood for tests related to menopause.
Current recruitment:

Bryan Brain Bank and Biorepository
Provider: Duke Department of Neurology, Duke Department of Pathology
Recognized as a premier biorepository for Alzheimer’s disease research, the Bryan Brain Bank and Biorepository provides investigators worldwide with access to longitudinal clinical data, high quality biospecimens, and histology services to support and advance basic and clinical research.
See what samples and data are available: ARENA Gateway: Neuropathology Brain Bank
The Duke/UNC Alzheimer’s Disease Research Center (ADRC) Autopsy and Brain Donation (ABD) program coordinates tissue retrieval from a variety of different sources, including participants of the ADRC Memory and Aging Study (MAS), the Bryan ADRC legacy cohort, and special autopsies from participants from underrepresented backgrounds or with unique genetic mutations or atypical clinical presentations. Skilled technicians of the Duke University Department of Pathology Autopsy Service perform the brain retrieval. The tissue is carefully preserved, meticulously catalogued, and stored in the Brain Bank and Biorepository.
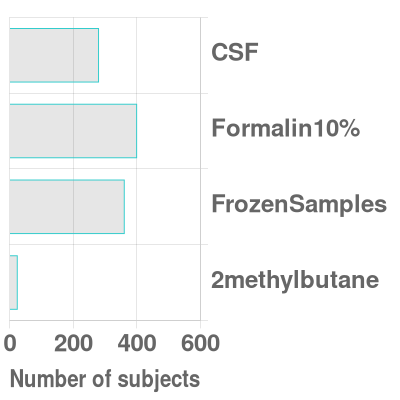
Brain Bank ResourcesThe Brain Bank collection includes over 1200 brain tissue (including 988 with fresh frozen tissue) and 500 postmortem CSF samples from 806 participants with AD, 215 with other neurologic diseases (Parkinson’s disease, frontotemporal dementia, amyotrophic lateral sclerosis etc.), and 234 healthy controls.
Brain Bank ServicesAs part of the NIH-funded Duke/UNC Alzheimer's Disease Research Center, it is our mission to share our resources and facilitate collaborations aimed at improving diagnosis and care for all people with Alzheimer's Disease and related dementias. Over the past 10 years, the Brain Bank has distributed over 2400 frozen and 600 formalin-fixed brain samples, 1600 slides, and 400 CSF samples to 110 investigators worldwide. The Brain Bank also provides histology and immunohistochemistry services, as well as consultation for investigators utilizing Brain Bank specimens for AD research. Investigators can request access to tissue and services through the Duke/UNC ADRC Resource Request portal. Tissue requests may be subject to review by the ADRC Executive Committee for scientific merit and resource availability.

Biospecimens Bank
Provider: Andy Liu, MD
The Duke University School of Medicine, Department of Neurology collects cerebrospinal fluid and plasma from patients in the Memory Disorders Clinic along with basic demographic and clinical data. These biospecimens are available to all researchers. We ask that all researchers submit a 1 page summary of the project before receiving biospecimens and acknowledgement of the use of this biorepository on all publications.
Contact: Sarrah Belhadj (Sarrah.belhadj@duke.edu)
See what samples and data are available: ARENA Gateway: Biospecimens Bank
Specimens
- CSF: ~300 samples (86 with AD; 209 with other neurologic diseases such as MCI, DLB and others; and 41 from healthy controls).
- Plasma: ~300 samples (81 with AD, 218 with other neurologic diseases such as MCI, DLB and others; and 14 from normal controls)

The Peri-Operative Neurocognitive Research Team (PORT) Studies
Provider: Miles Berger, MD, PhD
The PORT lab is interested in perioperative neurocognitive disorders, as well as mechanisms by which the APOE4 allele leads to long term neurocognitive decline. Available data includes samples from approximately 600 subjects collected before, during and after surgery. Data collected includes demographics, cognitive testing, and delirium screening, and samples of blood, plasma, and CSF supernatant and CSF cell pellets. Some subjects also have: fMRI data, intra-op EEG. Samples are stored in MSRB3.
Contact: Bethany Hsia (bjb15@duke.edu)
Website: Peri-Operative Neurocognitive Research Team Laboratory | Duke Department of Anesthesiology
- MADCO-PC (Markers of Alzheimer’s Disease and Neurocognitive Outcomes following Perioperative Care) was a prospective observational cohort study in older elective non-cardiac/non-neurologic surgery patients, which examined whether postoperative CSF tau increases are associated with the severity of postoperative cognitive dysfunction and changes in functional brain connectivity.
- The INTUIT study (Investigating Neuroinflammation Underlying Postoperative Neurocognitive Dysfunction, Delirium, and Brain Connectivity Changes) examined the role of CSF cellular and molecular markers of inflammation in the pathogenesis of perioperative neurocognitive disorders and associated brain functional connectivity changes.
- MARBLE (Modulating ApoE to Reduce Brain inflammation, deLirium and cognitivE dysfunction after surgery in older adults) was a phase II randomized, controlled, triple blind, dose escalation clinical trial of the ApoE mimetic peptide drug CN-105 in older elective non-cardiac/non-neurologic surgery patients at Duke. MARBLE tested the safety of CN-105 administration (its primary outcome), and secondary outcomes including: A) the feasibility of administering CN-105 q6 hours in these patients while they were hospitalized, B) its effect on postoperative delirium incidence and severity, C) its effect on 6 week postoperative cognitive function, D) its effect on intraoperative anesthetic-induced EEG patterns, and E) its effect on postoperative changes in CSF cytokine levels.
- Our current study, ALADDIN (Low Neurophysiologic Resistance to Anesthetics as a Marker of Preclinical/Prodromal Alzheimer’s Disease and Neurovascular Pathology, Delirium Risk and Inattention) is investigating the association between anesthetic induced EEG waveform patterns and 1) prodromal/latent Alzheimer’s Disease related brain changes, 2) prodromal/latent neurovascular disease markers, and 3) post-operative delirium and specific types of attentional and other cognitive deficits.
Completed Studies
The following resources are available from past studies:

Murdock – Memory & Cognitive Health Sub-Study (MHS)
Provider: Duke-UNC ADRC (PI: Dr. Welsh-Bohmer)
The Duke/UNC ADRC oversees the Memory & Cognitive Health Study, a sub-study of the MURDOCK Community Registry and Biorepository. The parent study was a longitudinal cohort recruited from a 20-Zip Code region in the Southeastern United States (U.S.) that is centered in the city of Kannapolis, NC and encompasses Cabarrus County, NC.
The MHS was a nested sub-cohort study of 1595 individuals who were 65 years of age or older in 2009. They received baseline cognitive assessment between 2009-2012 (Wave 1) and follow-up testing (Wave 2) from 2012-2016.
Contact: Kathleen Welsh-Bohmer (kathleen.welshbohmer@duke.edu), Brenda Plassman (Brenda.plassman@duke.edu). Sample stored at ODIN contact: David Layfield (david.layfield@duke.edu)
See what data is available: Memory & Cognitive Health Storefront
- MoCA
- CERAD Word List Learning and Delayed Memory test
- Trail Making Test Part B