A partnership with ADNI
The Alzheimer’s Disease Metabolomics Consortium has partnered with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to enable adding rich metabolomics datasets to all subjects enrolled in the ADNI study. These metabolomics datasets are meant to inform about metabolic failures across trajectories of disease and to complement genetic and imaging data within ADNI. In phase 1, we profiled blood samples from ADNI 1/GO/2 baseline using 8 complementary metabolomics and lipidomics targeted and non-targeted platforms. In phase 2, we profiled longitudinal samples from all ADNI studies covering over 5000 samples.
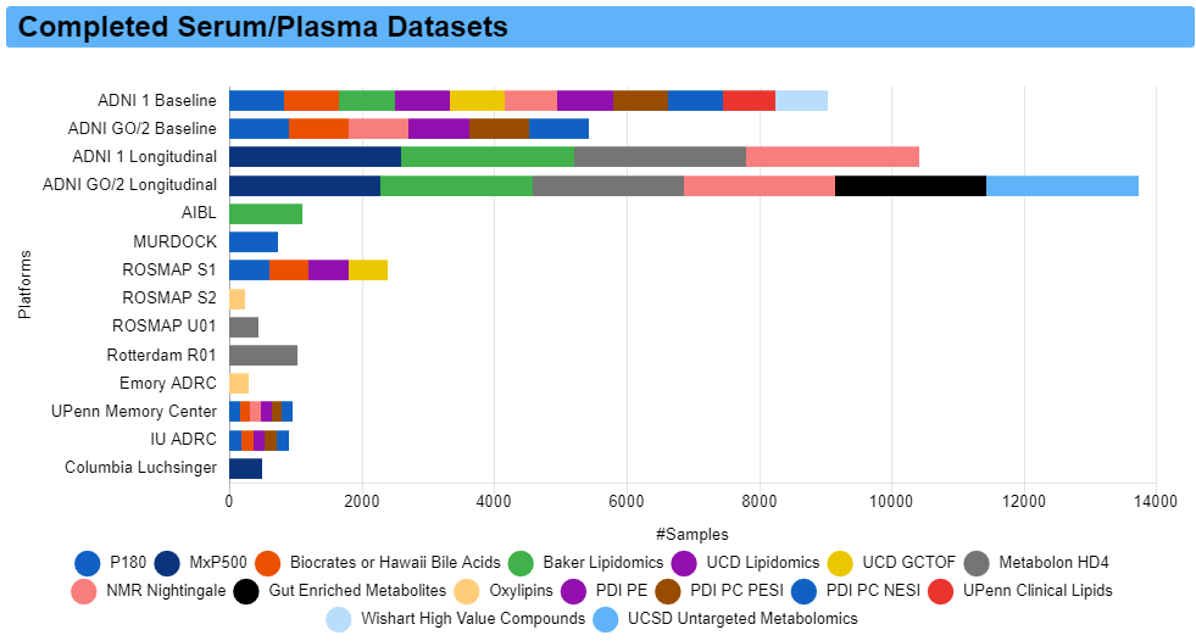
Below we provide background information on ADNI.
ADNI Informational Web Site: http://www.adni-info.org/
ADNI is a landmark public-private partnership that began in 2004 as a longitudinal, naturalistic study of older individuals with either Alzheimer’s disease, mild cognitive impairment (MCI), or no cognitive impairment at baseline enrollment.

ADNI has three overarching goals:
- To detect Alzheimer’s disease (AD) at the earliest stage possible and identify ways to track the disease through biomarkers.
- To support advances in AD intervention, prevention and treatment through the application of new diagnostic methods to apply at the earliest stages technically possible – when intervention may be most effective.
- To continually develop ADNI’s seminal data access policy and continuously improve and expand its unprecedented data sharing model.
The original ADNI study (now called ADNI-1) had the following enrollment targets:
- Alzheimer’s disease (N = 200)
- Mild cognitive impairment (N = 400)
- No cognitive impairment (N = 200)
ADNI-1 enrollment began in 2006, and provided for up to 5 years of follow up with annual assessments. Follow-up of surviving ADNI-1 subjects is continuing through subsequent ADNI projects.
ADNI-GO was funded to enroll an additional 200 subjects with MCI at an earlier stage than those enrolled in ADNI-1. The ADNI-1 MCI subjects are now referred to as having late MCI (LMCI); those enrolled in ADNI-GO are considered to have had early MCI (EMCI) at enrollment. ADNI-1 subjects received continued follow-up under ADNI-GO.
ADNI-2 was funded to enroll additional subjects with AD, LMCI, EMCI, no cognitive impairment, and a new group of subjects manifesting even more subtle degrees of cognitive manifestations either experienced subjectively or observed by others, termed significant memory concerns (SMC). ADNI-2 began in 2011. ADNI-1 and -GO subjects receive continued periodic follow-up under ADNI-2.

