Generally speaking, the Baugh lab is a c. elegans lab that observes developmental arrest (L1 arrest) in roundworms with various mutations under starvation to elucidate signaling pathways and gene regulatory mechanisms that control this arrest. These pathways and mechanisms mirror those of humans, and this research can be used to study the maintenance of homeostasis during development (adaptiveness), aging, and even cancer cell division and growth.
To describe my personal project, I first need to provide a bit of background information. When wild type (WT) c. elegans is starved, they enter a state of developmental arrest as larvae called L1 arrest. They remain in this state of arrest without growth for an extended period of time until conditions become more favorable, allowing them to recover and proceed with normal development. While they are under this developmental arrest, they do not age, so when they resume development, they also have a normal lifespan despite their previously arrested states.
This developmental arrest is controlled by insulin/insulin-like growth factor (IGF) signaling. There are two genes of special interest in this pathway: daf-2 and daf-16. daf-2 acts as a repressor to the transcription of daf-16, which regulates L1 arrest. Thus, with a daf-2 mutant, daf-16 becomes uninhibited, and the worm automatically goes into L1 arrest regardless of nutritional availability or presence of food. These mutants display a constitutive arrest phenotype, meaning that arrest always occurs no matter the circumstances. In contrast, when a daf-16 mutant faces starvation, the mutant shows an arrest defective phenotype and continues to grow and develop but dies quickly afterward due to lack of nutrition.
To determine whether larvae are arrested or not, we observe the appearance of the M cell lineage in L1 larvae. The M cell is marked by a transgene containing a GFP reporter, which causes it to glow green under a fluorescence microscope. During the L1 stage, larvae begin with a single M cell, and as they develop, the M cell divides over time to form up to 16 M cells. If properly arrested, all larvae of a particular strain should only have a single M cell. If even a single division is observed (>1 M cell), this indicates an arrest-defective phenotype in the strain.
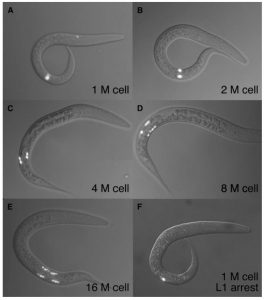
My personal project involves a collaboration of sorts with a lab at MIT. Researchers at MIT have identified arrest-defective phenotypes in ssu-1 and mrp-1 mutant worms under osmotic pressure (high salt) in comparison to WT worms, which properly arrest under high salt conditions. My job is to starve strains of ssu-1 and mrp-1 mutants and score their M cells to see if they are also arrest-defective under conditions of low nutritional availability. I also starve WT worms and daf-16 mutants alongside these two strains to serve as negative and positive controls, respectively. So far, I have not observed any M cell divides in these new mutant strains, which indicates that they are not arrest-defective when starved. However, I am still in the midst of replicating the experiment to determine whether my initial results are valid. There are many possible alternative reasons behind these results: low rate of division, slow development, or even just human error on my part among many other possibilities.
Additionally, I have been helping my mentor with one of her projects as well. Ivermectin is an antiparisitic drug that can be used to treat roundworm infections, and for c. elegans, ivermectin exposure paralyzes their pharynx, preventing them from eating food, although they can still sense it (fun fact: multiple people have accused us both of “worm torture”).
My mentor has observed that if worm larvae are exposed to ivermectin and food simultaneously for a period of time and then transferred to normal plates of food that are drug-free, they never develop and die quickly, although they are able to intake food. So far, she’s screened for mutagenized worms that might contain mutations of interest so she can better study the process behind why the larvae refuse to develop further. She’s already isolated various worm strains that have survived ivermectin exposure once, and for the past few weeks I have been helping her to put them through a second test to confirm that these strains have mutations that we’re interested in. Our next plan is to put the worms through complementation testing, which involves crossing worms together to see whether two strains contain the same gene of interest or different genes with the same effect. Eventually, we hope to elucidate the genes that regulate the mysterious halted development of ivermectin-exposed worms.
