A Promising result from Phase I and II Clinical Trials of a New SARS-CoV-2 Spike Protein Vaccine
Author: Beomyheok Lee
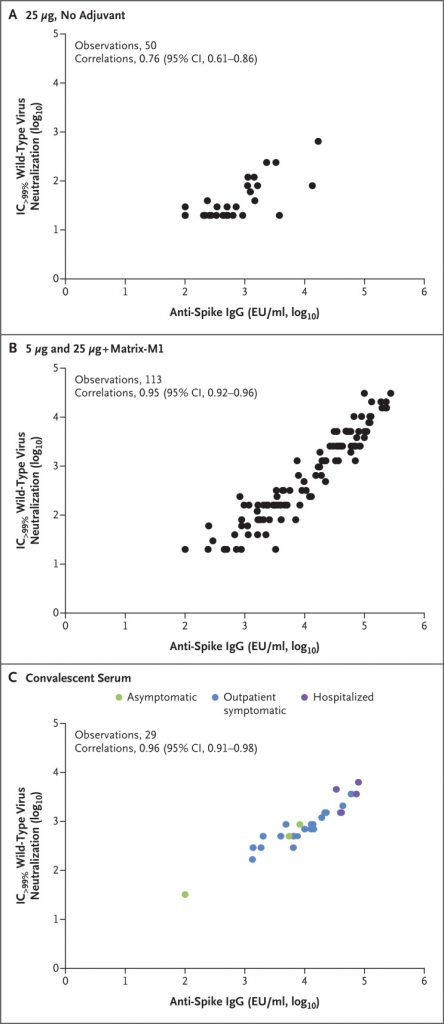
A phase I and II clinical trial of SARS-CoV-2 recombinant spike protein showed a promising result in inducing high immune response to SARS-CoV-2 viral spike protein. This vaccine aims to provide information about SARS-CoV-2 virus spike protein, which is involved in the virus’ fusion with human host cells, to human immune cells in order for prompt immune reaction to COVID-19 infection.
The figure on the top shows the correlation between the concentration of SARS-CoV-2 Anti-Spike IgG antibody produced by the vaccine and the antibody’s neutralizing activity on wild-type SARS-CoV-2. It shows that addition of adjuvant highly increased the neutralizing ability of the vaccine and showed similar performance to the antibodies generated by individuals that have recovered from COVID-19. The figure on the bottom shows the CD4+ T cell activity for each group of participants in the clinical trial. The figure demonstrates that the activity of T cell is highly dependent on the presence of the adjuvant Matrix-M1, and higher dosage of the vaccine showed better performance under the presence of the adjuvant (Retrieved from Keech C., Albert G., Cho I. et al., Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine, doi: 10.1056/NEJMoa2026920)
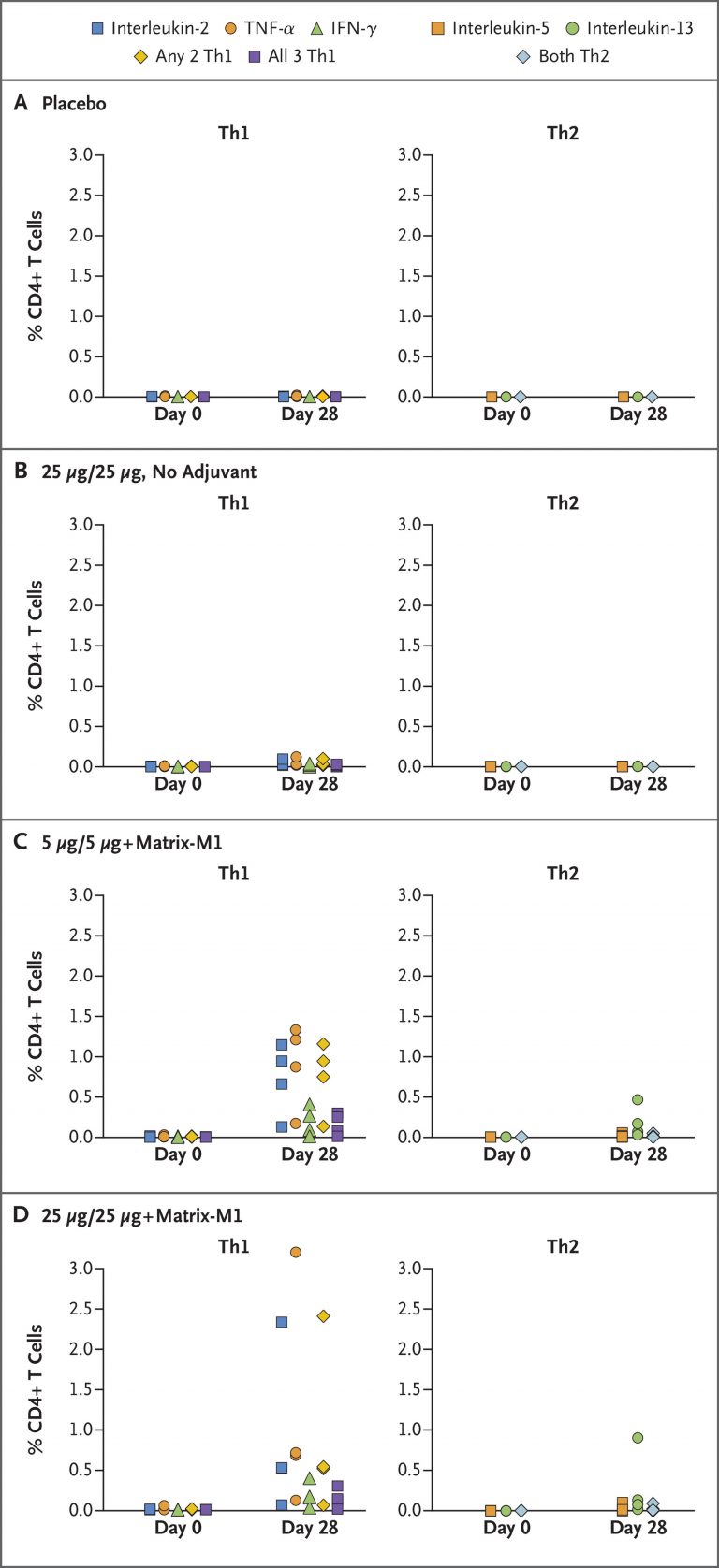
Researchers generated SARS-CoV-2 spike glycoprotein by utilizing Spodoptera frugiperda cell-expression system, which causes mutation at its cleavage site (682-QQAQ-685) to provide resistance to protease activities and two prolines (K986P and V987P) in order to stabilize and maintain the protein’s structure before it fuses with host cells. 131 people in total participated in the trial, with 83 participants receiving vaccines with adjuvants—micro agents that improve the performance of the vaccine, 25 participants receiving vaccines without adjuvants, and 23 participants receiving a placebo. No serious adverse events were noticed, and participants who received vaccines with adjuvants showed enhanced numbers and activity of IgG antibodies and CD4+ T in comparison to individuals who had recovered from COVID-19. However, the phase III randomized control trial with a larger group of participants is required to ensure the vaccine’s efficacy and safety.
References:
Figure retrieved from Keech C., Albert G., Cho I. et al., Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine, doi: 10.1056/NEJMoa2026920
Article title: Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine https://www.nejm.org/doi/full/10.1056/NEJMoa2026920