New Study for Vaccine Testing Immunogenicity and Safety of COVID-19 in Healthy Adults Aged 18 Years or Older
Author: Paula Gonzalez
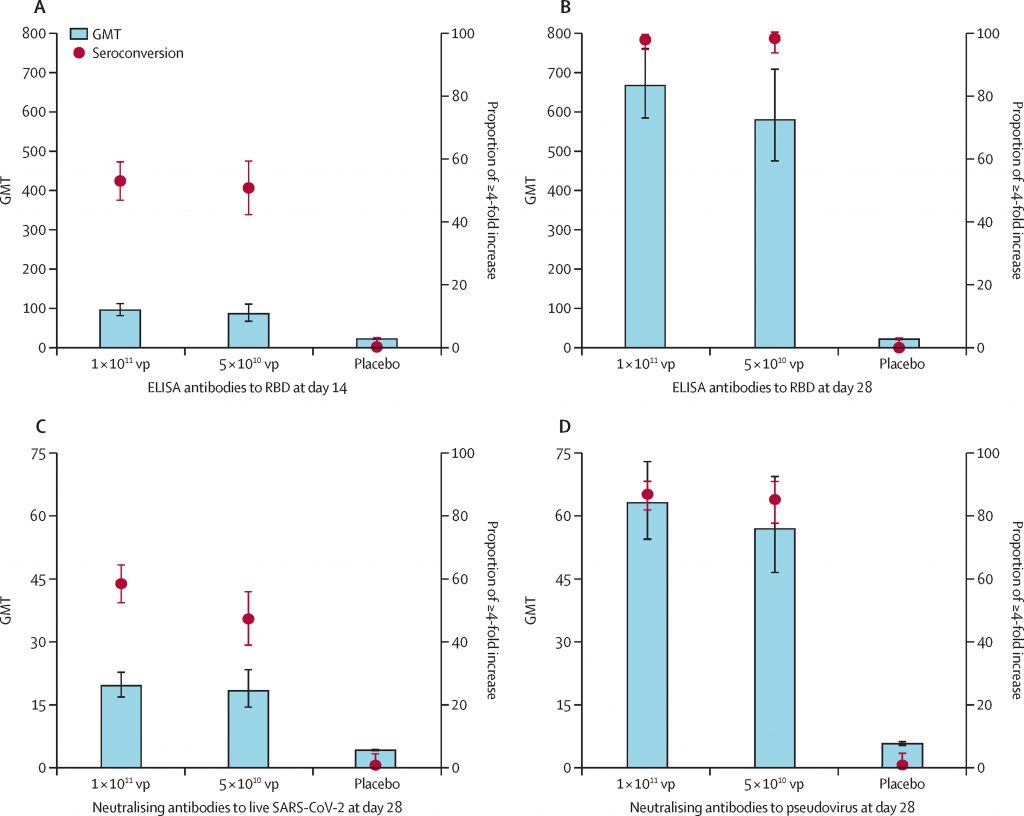
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 12.1 million cases of COVID-19 worldwide. The current pandemic has highlighted the need for effective solutions to reduce the burden. The first randomized controlled trial for assessment of the immunogenicity and safety of a candidate non-replicating adenovirus type-5-vectored COVID-19 vaccine was developed in Wuhan, China, with the aim of determining an appropriate dose of the candidate vaccine for an efficacy study.
This vaccine was tested on healthy adults aged 18 years or older who were infection free and were negative for previous SARS-CoV-2. This study provides evidence for the immunogenicity and safety of the Ad5-vectored COVID-19 vaccine in a larger population. Results suggest that a single-dose immunization schedule of Ad5-vectored COVID-19 vaccine is an appropriate treatment schedule for healthy adults. Older members of the population (ie, aged ≥55 years), showed to have significantly lower immune response, but higher tolerability to the vaccine, which indicates that an additional dose might be required to induce a better immune response in the older population. The primary objectives were to evaluate immunogenicity of the Ad5-vectored COVID-19 vaccine, and to determine a vaccine dose for a efficacy study. 603 volunteers were recruited and screened for eligibility between April 11-16, 2020. Baseline characteristics of the participants and the pre-existing Ad5 neutralizing antibody were similar across the treatment groups. Among the 508 eligible participants who consented to participate, 266 (52%) had high pre-existing immunity and 242 (48%) had low pre-existing immunity to the Ad5 vector. The interpretation of the results leads to the conclusion that the Ad5- vectored COVID-19 vaccine is safe and induces a significant immune response for the majority of recipients after a single dose.
References:
Figure retrieved from Feng-Cai, Zhu, Xu-Hua Guan, Yu-Hua Li. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial. https://doi.org/10.1016/S0140-6736(20)31605-6)
Article Title: Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31605-6/fulltext