Biopharmaceutical Drug Might Benefit Patients with COVID-19
Author: May Thongthum
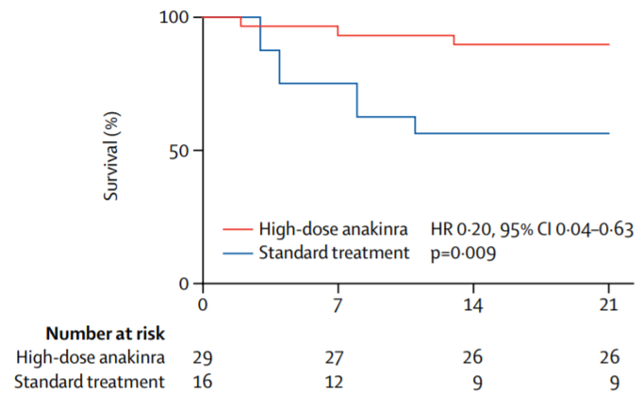
A retrospective cohort study published in The Lancet Rheumatology on May 7th has reported that intravenous administration of high-dose anakinra, a recombinant interleukin-1 receptor agonist, on patients with COVID-19 and acute respiratory distress syndrome (ARDS), which is the main cause of death for COVID-19 patients as it leads to respiratory failure, was “safe and associated with clinical improvement in 72% of the patients”. Since current pandemic outbreak conditions mean patient numbers can far exceed ICU capacities, interleukin-1 blockade treatment via anakinra, on top of standard care and non-invasive ventilation, might help address such clinical and capacity-related problems.
The study reports a cumulative survival rate of 90% in the group treated with high dose anakinra, standard treatment and non-invasive ventilation compared to 56% in the group that only received the standard treatment and non-invasive ventilation after 21 days. While this study has several limitations regarding the patient population, long-term outcomes and need for extensive follow-up in order to achieve more definitive conclusions, the study is clearly promising.
References:
Cavalli, G., De Luca, G., Campochiaro, C., Della-Torre, E., Ripa, M., Canetti, D., … & Dagna, L. (2020). Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology, 2(6), e325-e331.