Research projects in our laboratory are focused on:
The Innate Antiviral Response
Adaptive immunity, such as tumor immune surveillance, requires stimulatory innate signals, e.g. type-I interferons (IFN), that trigger processes leading to antitumor T cell immunity.
The ultimate line of defense against RNA viruses are the cytoplasmic viral RNA sensors MDA5 and RIG-I, which mediate downstream signals yielding antiviral type-I IFN responses. The Picornavirus family has an innate footprint distinct from all other RNA viruses, because host innate defenses are orchestrated principally by MDA5, rather than RIG-I.
We discovered that the unusual non-cytopathogenic PVSRIPO:host relationship generates peculiar spatiotemporal patterns of type-I IFN release that are optimal for priming of adaptive antitumor immunity:
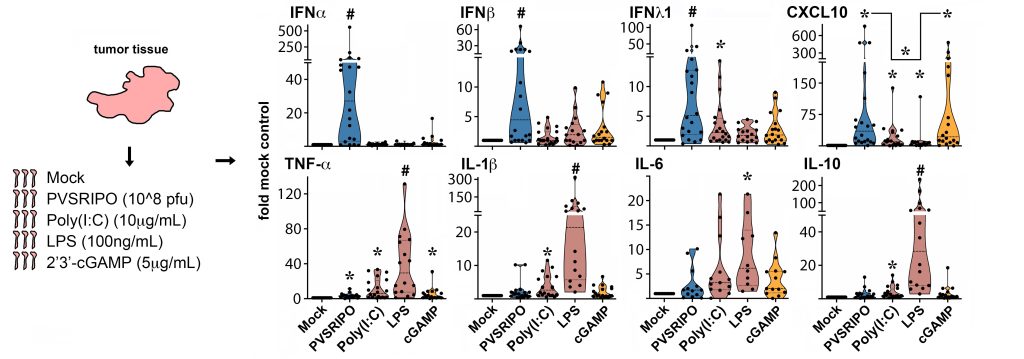
The Innate Signature of PVSRIPO. PVSRIPO infection of ex vivo glioblastoma patient tumor slices—undissociated tumor fragments—reveal its unique innate signature relative to agonists of toll like receptor 3 [poly(I:C)]), -4 (LPS) or cGAS/STING (cGAMP). From Brown et al. (Nat Commun, 2021, PMCID: PMC7994570).
We are studying the molecular mechanisms of MDA5-orchestrated IFN signaling, e.g. elucidating virus:host relations dictating the innate antiviral defense, or resolving innate signaling cascades responsible for sustained type-I IFN release.
Deciphering Adaptive Antitumor Immunity.
Tumor immune surveillance relies on myeloid antigen presenting cells (APCs) and their capacity for cross-presenting tumor antigens and prime antitumor T cells. Poliovirus naturally targets its receptor—the immune checkpoint molecule CD155—on myeloid APCs. The main polio reservoir is the lymphatic system; recent evidence suggests that poliovirus may reach this reservoir by targeting peripheral APCs that home to lymph nodes draining the infection site.
PVSRIPO infection of macrophages/dendritic cells (DCs) is non-lethal, yielding accentuated MDA5-orchestrated sustained type-I IFN release. We are investigating how PVSRIPO infection shapes myeloid APC phagocytic activity, lymph node homing, antigen presentation, T cell priming, and adaptive antitumor CD8+T cell immunity:
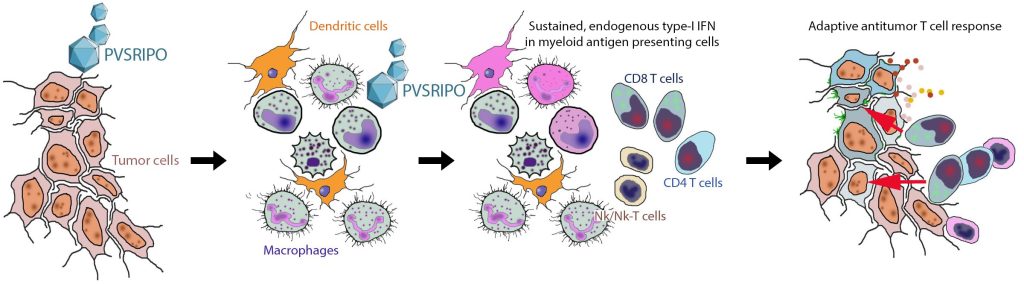
PVSRIPO Primarily Targets Myeloid Antigen Presenting Cells. CD155 is widely expressed in solid tumor neoplastic cells: PVSRIPO infects neoplastic cells and myeloid APCs (macrophages/DCs) in tumors. Myeloid cell infection is pivotal for immunotherapy, as it elicits endogenous, sustained type-I IFN release for proper stimulation of adaptive antitumor immunity. See Brown et al. (Sci Transl Med, 2017, PMCID: PMC6034685).
Antigen presentation, and durable antitumor immunity in the brain.
The principal focus of our clinical translational work are malignant brain tumors, where PVSRIPO immunotherapy may hold unique potential. This has been demonstrated in clinical studies in patients with recurrent glioblastoma:
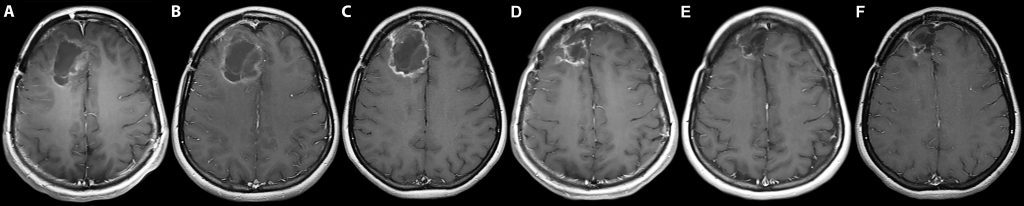
PVSRIPO in Clinical Trials. From Desjardins et al. (N Eng J Med, 2018, PMCID: PMC6065102): A. Baseline MRI. B. Tumor expansion 2 months after single intratumor PVSRIPO infusion. C. Initial tumor contraction 6 months after the infusion. D., E. Gradual remission at 12 and 24 months, respectively. F. Contraction of the resection cavity and the tumor 58 months after the infusion.
The brain has an anatomically and functionally unique immune landscape with an intriguing system of lymphatic drainage: ‘glymphatic’ transport provides for DC homing to cervical lymph nodes via meningeal lymphatic vessels. Microglia, together with CNS-associated macrophages (CaM), constitute a unique, specialized tissue resident myeloid compartment.
PVSRIPO targets brain-infiltrating APCs, microglia and CaM and induces global type-I IFN dominant neuroinflammatory phenotypes in the CNS. We are exploring how viral targeting of diverse myeloid compartments in the brain contributes to tumor antigen cross-presentation and T cell priming.