Grant Submission Process - An Overview
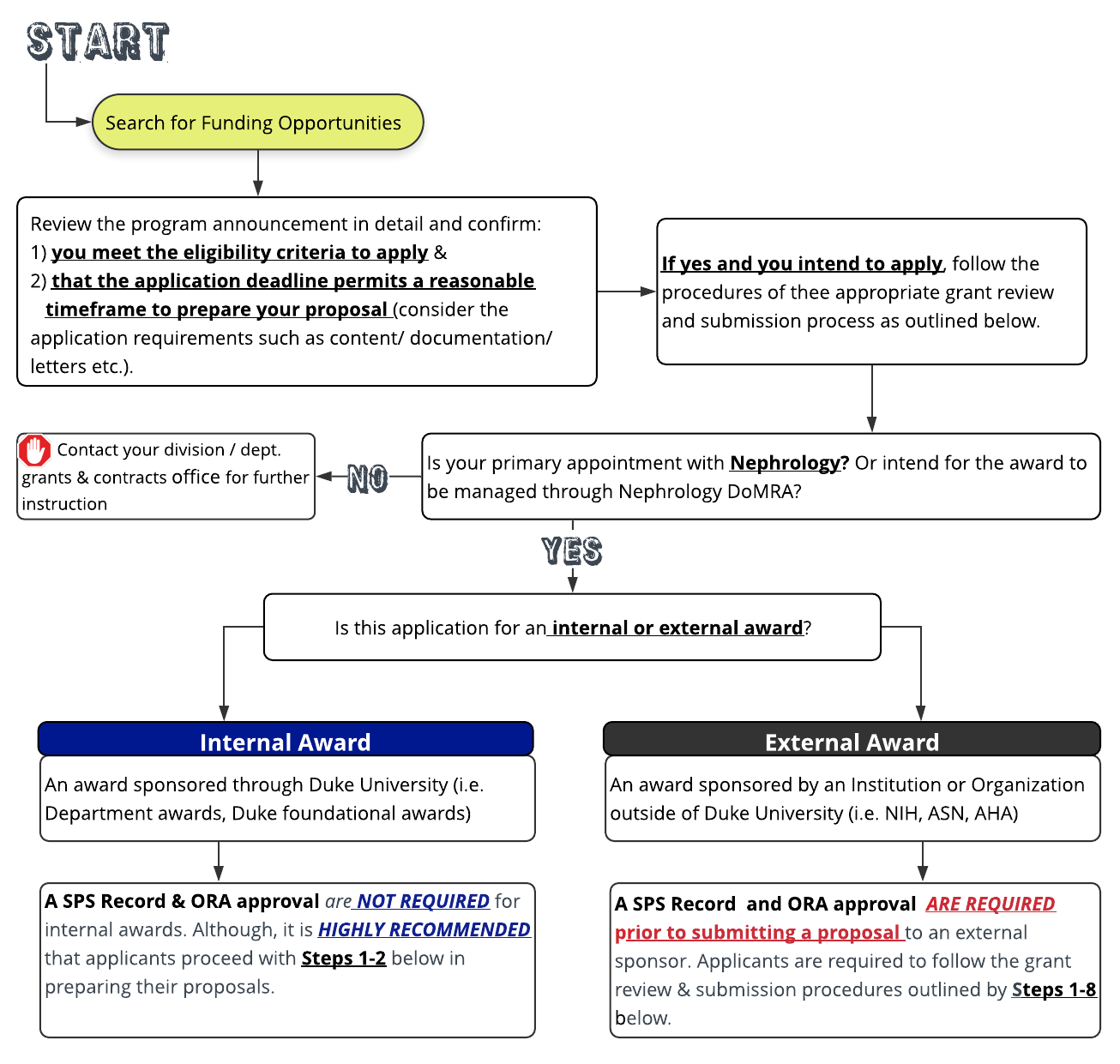
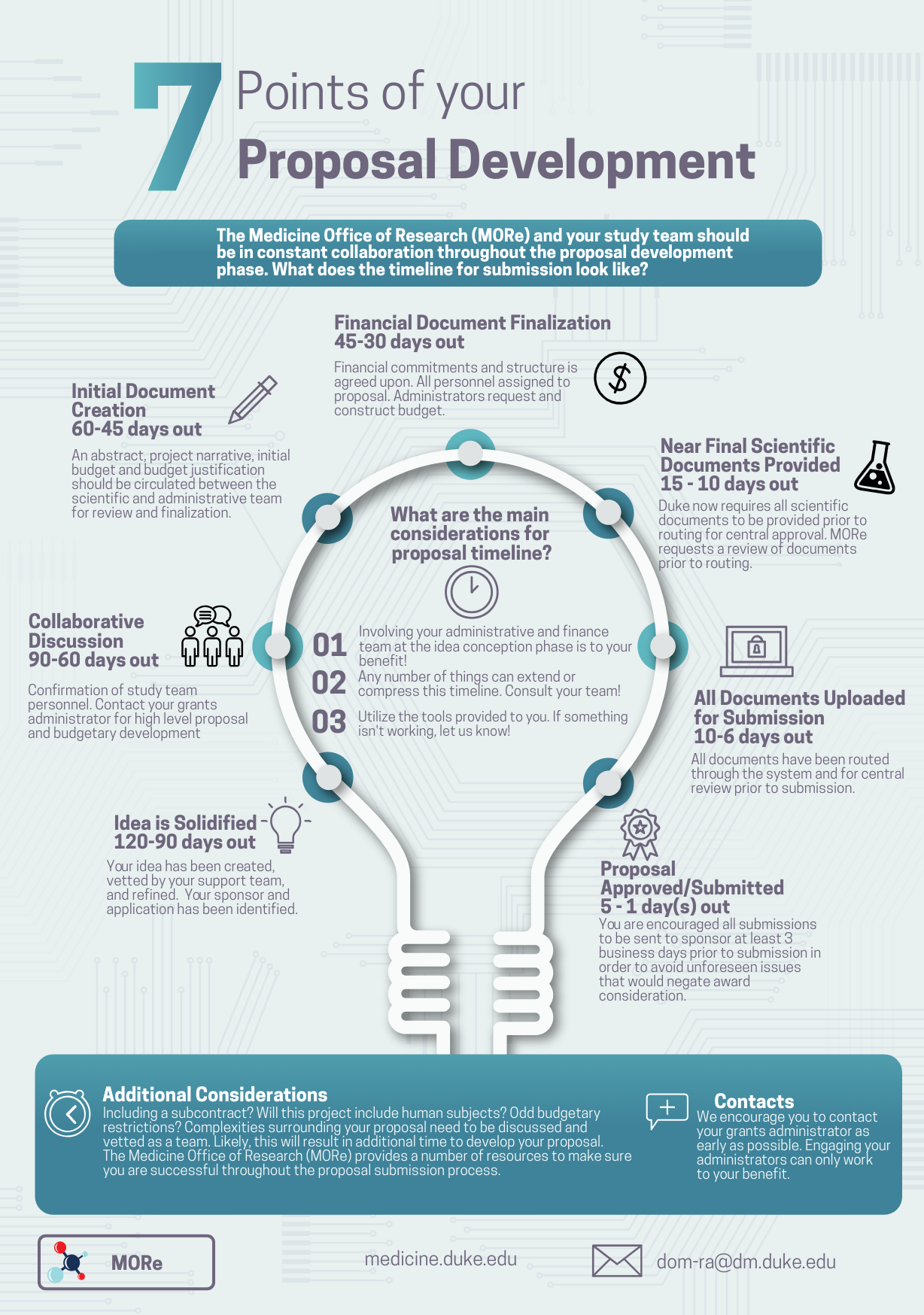
In planning to submit an award application it is important to recognize early that there will be significant overlap in the timelines for developing your proposal, routing for internal review, and submitting the final application to the sponsor.
**Therefore it is crucial that applicants have a firm understanding of the grant submission process, new ORA review policies (effective July 2019-onward), application requirements (FOA and special research considerations) and when and how to prepare these components.
**It is recommended that early on in the process applicants review both the ‘Grant Submission Process’ page (here) and sub-pages under the ‘Develop Proposal’ tab and consider these in parallel to plan for a timeline that works succinctly between the two.

At least 6 weeks prior to the sponsor deadline – Complete the online New Proposal Intake form (linked below). This feature will automatically alert the grants management team of your intent to apply.
IMPORTANT NOTE: Download the most up to date version of the Nephrology Budget Template (linked here, as well on the budget and cost resources page) vs. the proposal intake form as the updated version is not guaranteed to be available in the intake form at the same time. We are working to have these synced in the near future to avoid any confusion.
Nephrology Grants Manager-Rhonda McCloud or Associate Director of DoMRA Adam Lawler and the assigned grants administrator will email you within 2-3 business days to schedule a pre-award meeting (or call) to review the proposal requirements, build out the budget and discuss the timeline for submission.
Note: As part of the Nephrology Division’s new intent to submit process, the new electronic Proposal Intake Form will replace the Excel based ‘New Proposal Form’ (referenced here), which will no longer be required as part of the SPS record.
Plan to schedule and attend a pre-award meeting with your grant management team within 7-10 days of completing the New Proposal Intake Form (Step 1).
Note: If you have no received a follow up email from Adam Lawler within 2-3 business days of submitting the proposal intake form online – contact him directly adam.lawler@duke.edu, (919)-684-5407 to account for any unforeseen circumstances or system errors that may disrupted this initial line of communication.
|
Providing these documents as they are completed in the weeks leading up to this deadline will allow your grants manager time to review, identify errors and suggest corrections prior to submitting for ORA review.
Example ‘Non-technical/scientific sections’ include (but are not limited to):
Duke (Primary site / parent award recipient)
- Budget
- Budget Justification
- Letters of Support (LoS) – Department Chair and Division Chief LoS)
- Letters of Commitment for Institutional/ Departmental/ or Divisional Support
- Biosketches (key personnel and other significant contributors)
- Facilities and Resources Page
- Equipment Description
If proposal includes Subawards
- ***All required subaward documentation
- LoS , NIH biosketch, and Statement of Work (SoW) for each key personnel named on the subaward
- PHS 398 PI Checklist
- PHS 398 Signed Facepage
- Subrecipient FCO! certification form
- Subaward Budget (R&R Budget packet)
- Budget justification
- Facilities and Resources page (including equipment description where applicable)
Per the new ORA internal deadline policy – “A complete and compliant application must be received by ORA no later than 5 business days prior to the sponsor’s deadline (the ‘Internal Deadline’). If an application is to be submitted via Grants.duke, all components must be uploaded by the internal deadline. If an application is to be submitted using a sponsor electronic system, ORA must also have access to review and submit the application using the sponsor’s system by the internal deadline. Failure of the PI or his/her designee to grant ORA access to the final application may result in the application not being submitted timely or not being submitted at all.”
Note: A late submission is defined as a SPS record received by ORA after this specified deadline regardless if the remaining documents were provided to the grants manager 5 minutes before the deadline. Therefore be sure to allow your grants manager sufficient time to prepare, review and route the SPS record to ORA. Be sure to account for delays and/or other scheduling complications when an internal deadline falls on a Monday (8:00am). Such logistics and planning should be clearly communicated with your grants administrator.
Once the remaining sections are provided(6 business days prior), your grants manager will then route the SPS record to ORA for review by 8:00am 5 business days prior to the sponsor deadline.
| Following the 5 Day Application Review Deadline policy (effective July 2019): ‘The “technical” or “scientific” sections may be submitted in near final form to allow additional time to finalize these sections prior to submission, through it is expected that these sections will differ only slightly updates to data tabled, references cited or bibliography)’. | ||
| Example technical or scientific sections include (but are not limited to) | ||
| ||
|
If the SPS record is not routed to ORA 5 days prior to the sponsor deadline, a grant application waiver request will be required in order to proceed with ORA review for submission approval.
|
|
|
If the waiver is approved, ORA will make make every effort to ensure a successful submission. Applications that must go expedited review will be reviewed after the fact and may be withdrawn if non-compliant with institutional or sponsor requirements.
Examples of errors which would prompt a submission to be withdrawn include the following:
- Placeholders
- Non-compliance with institutional policy – e.g. publication restriction without exception letter, cost sharing, improper use of name/logo/trademark
- PIs are no longer affiliated with or physically located at Duke
- PI/Institution does not meet the eligibility criteria
- PI not selected to submit proposal (limited submission)
- Falsified information, data or signatures.
The 5 day review policy does NOT apply to the following items listed below, however Nephrology applicants should communicate intent to submit documents below and review (steps 1-2 of the grant submission process).
- Letter of Intent to Apply
- Pre-Proposal (sponsor may require a brief 1-2 page narrative of the proposed project idea and total cost estimated (not a detailed budget)
For a better understanding of the ORA review process, what to expect, and the errors to avoid reference the Duke ORA links below:
Once you address the corrections and provide the updated sections of the application to your grants manager, your grants manager will then update the SPS record and reroute to ORA to verify the changes.
Note: In all cases, ORA reserves the right to require changes not listed on the ORA website, by sponsor requirements and changes to institutional police. As such be proactive in confirming ORA request which you may have questions or concerns about with your grants research administrator. The Nephrology DoMRA team will assist in the communications with ORA and provide excellent details, clarification and feedback on the requirements
- If additional revisions are required, repeat steps 6-7.
OR
- If ORA provides subsequent approval of the application, proceed with step 8.
When submitting an application to a federal sponsor in Grants.Duke (Duke’s electronic system to routing to Grants.gov) it is important to note the following details in planning for an early submission (2-3 days prior to the sponsor deadline)
- Grants.gov validation can take up to 48 hours. Your application is on time if Grants.Duke (Duke’s electronic system which routes to Grants.gov) timestamps it by 5:00pm your institution’s local time on the receipt date listed in the FOA.
- Commons validation can take up to 24 hours.
- After passing Common’s validation, you get up to 2 business days to use the viewing window to check the application image. If you take no action, it moved forward to NIH referral officials at the end of the 2 days.
- If there are problems with your application and you have time before the deadline, you can fix the problems and submit again.
NIH’s Center for Scientific Review (CSR) check your application for administrative and formatting requirements
CSR also assigns the application to an NIH Institute or Center and a review group
Check the CSR Peer Review Videos to know more about the review process
2-step peer review process by outside scientific experts
- 1st step: initial peer review by a study section organized by NIH’s Center for Scientific Review or NIH Institute
Initial peer review meeting take place 3-4 months after you apply
Peer review results in a numerical value, called overall impact score, indicating your reviewers’ judgement of the likelihood that your project will have a powerful impact on its area of science. Your overall impact score is the most important factor for a funding decision
Streamlined applications are those that reviewers consider to be noncompetitive among the pool of applications received. Though they do not receive an overall impact score, they receive a summary statement with the reviewers’ critiques but no resume of the discussion
- 2nd step: Each NIH Institutes/Center Advisory Council approves the study section’s recommendations
Track your Application
You can track your application, view summary statements and scores, send just-in-time information using eRA Commons
- You should see your application’s assignments in the Commons within 2 weeks after you submit. If not, contact NIH CSR.
- 30 days before the peer review, you should see the review roster posted in the Commons.
- Your application undergoes initial peer review
- Within 3 days after the review, you should see your score in the Commons
- Within 30 days after the review, you could see your summary statement in the Commons
Click here to see more about Timelines for Assignment, Review, & Funding
Plan for potential revisions/resubmission
Click to see Options if Your Application Isn’t Funded
Unlimited resubmissions are now allowed for NIH
- A0: New
- A1: Resubmission, A1 is only allowed once for the same project!
So, you can try either
A0 (new, original)–>A1 (resubmission)–>A0 (new)–>A1 (resubmission) or A0 (new, original)–>A0 (new)–>A0 (new)–>A0 (new)
- For problems you can fix, Revise and Resubmit an Application (A1)
- If you choose A1, an Introduction is also required and next reviewers can see the A0 Summary Statement
- For problems you can’t fix, or if you’ve used your resubmission opportunity, do one of the following:
- Create a New Application: You may pursue a similar idea or choose a new topic (A0)
- Apply Outside of NIH: Another organization may be able to fund your research