There are three considerations when reviewing budgetary considerations:
- How much do I have per year?
- Are there specific budget items which are not supported (including F&A/indirects) in the FOA?
- Are there specific budget items which are required per the FOA?
Overview On NIH Budget Types
The NIH uses 2 different formats for budget submission: Modular and Non-Modular. If it is not readily apparent which budgetary format your project will utilize in the FOA, consult your grants administrator.
The application forms package associated with most NIH funding opportunities includes one of two budget forms—(1) R&R Budget Form; and, (2) PHS 398 Modular Budget Form. To determine whether to use a detailed versus modular budget for your NIH application, see the flowchart below and always refer to the FOA and appropriate SF424 application instruction packet for guidance on which Budget form is required.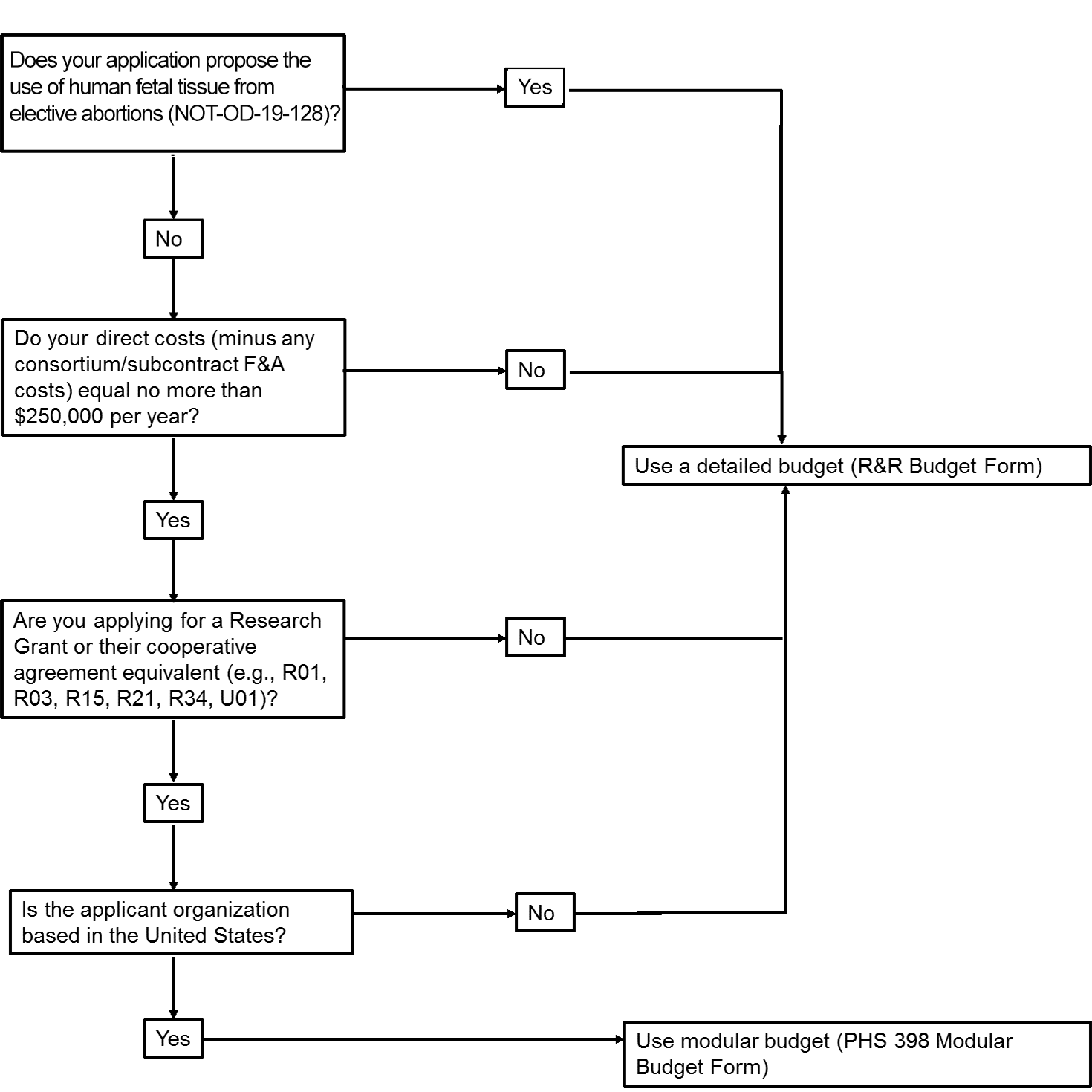
Note that you will not be required to complete this form. Duke grants platforms and/or grants administrators will complete this on your behalf once a budget is finalized.
Modular Budgets
A modular budget format can be used when requesting up to $250,000 of direct costs per year (excluding consortium F&A). In the format, you are required to submit funding in modules of $25,000. If you have a consortium, they must submit with a total to the nearest $1,000. As a rule of thumb, if your FOA is a “parent”, they will typically utilize the modular format.
Creating a modular budget:
- In order to determine how many modules you should request, subtract any consortium F&A from the total direct costs, and then round to the nearest $25,000 increment.
- Please Note: If the prime application is being submitted to NIH utilizing a modular budget, the subaward site will need to prepare a detailed budget for internal Duke purposed.
TIP: As Duke is under additional administrative oversight, the NIH is requiring detailed budgets at just-in-time (JIT). Creating a detailed budget for your own institution’s use including salaries, equipment, supplies, graduate student tuition, etc. for every year of funds requested is important to have when planning your proposal, calculating your F&A costs base and writing your justification, and for audit purposes. Additionally, it will help expedite the award issuance at JIT.
A modular budget justification should include:
- Personnel Justification: The Personnel Justification should include the name, role, and number of person-months devoted to this project for every person on the project.
- Consortium Justification: If you have a consortium/subcontract, include the total costs (direct costs plus F&A costs), rounded to the nearest $1,000, for each consortium/subcontract. Additionally, any personnel should include their roles and person months; if the consortium is foreign, that should be stated as well.
- Additional Narrative Justification: Additional justification should include explanations for any variations in the number of modules requested annually. Also, this section should describe any direct costs that were excluded from the total direct costs (e.g. equipment, tuition remission, patient care costs) and any work being conducted off-site, especially if it involves a foreign study site or an off-site F&A rate.
See the NIH Modular Research Grant Applications page and the NIH Grants Policy Statement for more information.
A Note About SBIR/STTR:
The modular budget format is NOT accepted for SBIR and STTR grant applications.
Detailed Budgets
A detailed budget is the most common type of budget when submitting for sponsored research support. This line-by-line format includes: salary/fringe, supplies, travel, publication costs, subaward/ consultant etc. NIH uses a detailed budget format (R&R Budget) to request more than $250,000 in direct costs per budget period or when specified in the FOA.
Refer to the main budget & cost resources page below for further instruction on how to prepare a detailed budget and budget justification.
Preparing a Detailed Budget
Use the Nephrology Budget Template to plan and build out a detailed budget for any proposal. Review the budget sections below and determine what budgetary components are applicable to your project and specific aims. Follow the instructions and guides for each respectively and a draft Budget (Nephrology Template) and Budget Justification This can be completed at time of proposal intake or in concert with your assigned Grants and Contracts Administrator.
Note: The draft budget (Nephrology template) will provide an estimated calculation for your project costs. Your GCA will input the final calculations and discuss with you prior inputting the information into the Sponsored Project System (SPS), which will then populate as part of your application in Grants.Duke and the SF-424.
**Updated 3.17.2023
**New Federal and Non Federal Fringe Rates Per May 2022 Memorandum. New NIH Salary Cap effective Jan 1,2023. New NIH Postdoctoral stipend levels 2023.
Direct Costs
Personnel
Refer to the NIH- NIAID site for team considerations and recommendations for building a strong and effective team.
A. Senior/Key Personnel
The PD/PI and other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way to the scientific development or execution of the project. Typically, these individuals have doctoral or other professional degrees.
Ask yourself the following: (1) am I paying this person for their work and (2) do I want their biosketch included in the proposal. If both are yes, this would constitute key personnel.
B. Non-Key Personnel
Personnel who are important to the lab, but do not direct the science in a meaningful way should be considered non-key personnel. This includes, but is not limited to, post doctoral associates, lab technicians, and clinical coordinators.
Ask yourself the following: If this person were to leave the project, could they be replaced in or would the absence of their technical skills cause a major setback to the project? If no, then consider this person non-key personnel.
C. Other Significant Contributors (OSCs) are individuals who have committed to contribute to the scientific development or execution of the project, but are not committing any specified measurable effort to the project. These individuals are typically presented at “zero percent effort or zero person months”. Individuals with measurable effort may not be listed as (OSCs), but should include the biosketch.
Ask yourself: Is this person considered a consultant, adviser, or mentor? Are they working without pay? If the answer is yes to both, then consider this person an OSC.
Additional Designation FAQ’s:
http://grants.nih.gov/grants/policy/senior_key_personnel_faqs.htm
Key Considerations
- Your colleagues and grants administrators can help!
- Communicate to your collaborators (other named key personnel) early of your intent to submit and discuss/agree upon the % effort they will apply to the project.
- All personnel from dedicating effort to the project should be listed on the budget
- Salaries should be represented off of a university base salary (e.g. not inclusive of PDC/VA)
- Duke strongly encourages a 3% increase for all personnel salaries in outlying years
- TBD (To be determined) personnel may be budgeted for non-key personnel. This applies to cases in which only if the award is funded, would personnel be recruited to apply effort to the project.(Ex. hiring a new lab technician, post doc/student, or statistician / statistical programmer, etc.–and who identity is not yet known.)
- For clinical research proposals, remember to work with your GCA and CRU to identify if CRU support is needed and at what level of effort (Clinical research coordinators (CRC), regulatory coordinators (RC), registered nurse practitioner etc)
Additional Considerations
- Clarify if you/your colleagues are discussing effort commitments in total professional effort (TPE) or university effort.
- The NIH and many other organizations enforce an Executive Level Salary Cap
- Post doctoral associates/scholars will utilize the required federal stipend levels
- Students will be provided a stipend based on graduate school rates
- SOM faculty utilize a 12 month calendar, campus faculty 9 month/3 month calculation
How Do I Obtain Salary information?
Duke University does not publicly provide salaries without a formal request. Please work through your grants administrator to obtain all salary information
| 1) Consider using a table at the top of your personnel section to easily clarify name, role, and commitment to the project. |
| 2) Consider using subheadings to clearly differentiate personnel as either Senior/Key Personnel, Non-Key Personnel or Other Significant Contributors. |
| 3) List all personnel by name, followed by their project role and level of commitment (effort in person calendar-months). |
Note: Only non-key personnel may be listed as ‘To be determined’. If a name is not available, utilize the “TBD” tag in lieu of the name in the justification.
| 4) Personnel justification should be descriptive in the aim(s) the individual will be supporting as well as ancillary activities that support the project (e.g. manuscript development, study team meeting participation, presentation of information, etc;). |
Consultant Costs
| Consultant | Vendor | Subcontractor | |
| Work Performed |
|
|
|
| Additional Budget Elements |
|
|
|
| Additional Considerations |
|
|
|
Consultant
Consultant designation is appropriate for non Duke employees – Duke employees typically MAY NOT BE consultants (role for Duke employees can be listed as project personnel). Note for potential exception provided in the note below.
Note: In unusual situations, a person may be both a consultant and an employee of the same party, receiving compensation for some services as a consultant and for other work as a salaried employee as long as those separate services are not related to the same project and are not charged to the same project. For example, consulting fees that are paid by an educational institution to a salaried faculty member as extra compensation above that individual’s base salary are allowable, provided the consultation is across departmental lines or involves a separate or remote operation and the work performed by the consultant is in addition to his or her regular departmental workload. See NIH Salaries and Wages/Intra-IHE Consulting for further information. Work with your GCA early in the application process to identify if such circumstances apply. The GCA will work to obtain the necessary documentation and information to formalize the agreement. |
Your ability to set the expectation will often be followed by the consultant.
- Make sure your scope for the consultant is limited and well defined.
- Consider the amount of hours you will reasonably need your consultant.
- Scope of Work
- Letter of support
- FCOI – Consultant form
| 1. State the Consultant’s primary affiliation and expertise |
| 2. Describe the services to be performed by the consultant(s) and at what frequency. |
| 3. Provide justification for the need for these specific consultant services in the context of the work being proposed. |
| 4. Provide an itemized breakdown of the funds requested. |
- Cost calculations should be based on the formal agreement with the consultant and should include the following;
- hourly/daily rate of pay
- number of hours/days of anticipated consultation
- travel, per diem, and other related costs for each
- Cost calculations should be based on the formal agreement with the consultant and should include the following;
Supplies
| Refer to the following infographic- “Research Supplies & Other Expenses – Budget Strategy” for how to accurately estimate supply and other expense costs. |
General points (for detailed instructions/examples refer to infographic above)
- Supplies must be necessary for completion of project and consistent with research plan
- Estimated supply costs should be precisely calculated and planned out.
- Outline experiments and group supplies into categories
- Start with the most expensive supplies in each category and perform cost calculations for each
- Categories that include costs less than $1,000 do not have to be itemized, however for supply categories >1,000 you will need to provide a general breakdown of these expenses and provide further detail in the budget justification.
- General office supplies and computers, printers (and related accessories) are NOT allowable expenses and should NOT be included.
| Example | |
Award Year 1 | |
| Supplies | Cost |
| Reagents & Kits (Antibodies and cull culture, RT PCR. RNAscope probes, genotyping, DNA&RNA Isolation kits etc) | $ 25,000 |
| Immunoassays (FGF23 and TNFr Elisas, Luminex Multiplex Panel assays) | $10, 000 |
| Basic Lab Consumables (plastics, glassware, animal surgery consumables/supplies) | $ 5,000 |
Experimental Animals | $900 |
Subtotal Supplies | $40,900 |
Justification language should include
- Should include general categories for essential supplies such as reagents, assays & kits, consumables, animal costs etc,
- Note: ‘Animal Costs’ involve the direct purchase of experimental animals and/or supplies purchased directly from a vendor (i.e. dietary chow) and are always designated under ‘Supply cost’. This is separate from the animal care and maintenance cost/services listed under ‘Other Expenses’
- Should include the amount requested for each category
- Should include how cost was derived (if applicable)
- Should include justification of the supplies in the context of the research plan (reference to specific experiments proposed, or work in context of the specific aims)
- Categories less than $1,000 do not need to be itemized, however you should provide details of the expense as part of the justification
| Example |
Supplies $40,900 is requested in each year of the award to support the projected cost of laboratory supplies and materials. This includes $25,000 in kits and reagents specifically for RT-PCR reagents, mouse genotyping, western blotting, RNA hybridization kits, standard histology staining kits and chemicals, antibodies, and cell culture media; $10,000 in immunoassays for measuring murine creatinine, albumin, FGF23 and TNFr; $5,000 in laboratory consumables, primarily plastics and glassware used in bench-work experiments, sample collection and processing, cell culture and animal surgical procedures; and $900 for the purchase of 15 experimental B6 mice from Jackson Laboratory ($60 per mouse). |
Other Expenses
| Refer to the following infographic – “Research Supplies & Other Expenses – Budget Strategy” for how to accurately estimate supply and other expense costs. |
Non-supply costs – Other Direct Costs
- Must be necessary for completion of the project and consistent with the research plan
- Other Direct Costs may include but are not limited to;
- Subject costs (payment,meals, parking) – compensation for subject participation. Distinct and separate from patient care costs.
- Vendor / Core Services
- DLAR Animal Care and Maintenance – Follow the current approved per diem rates – DLAR FY20 Per Diem
- Computer Software (statistical, graphing, or manuscript software specifically for use on the project)
- Publication Costs
- Estimated supply costs should be precisely calculated and based on a quote obtained or price is based on previous work with similar scope
- Should not include unallowable or indirect costs (i.e entertainment, renovations. general office IT or administrative support services etc)
- Refer to FOA to determine if other direct costs restrictions apply
Other Project Related Expenses
In the budget justification, indicate other project related expense categories (direct costs), including an amount for each category and brief justification for each.
Justification language should include
- Should include specific deliverable or good being requested
- Should include how cost was derived (if applicable)
- May include recognition of previous work with organization (signifying confidence)
- Should include confirmation that a quote for cost was obtained or price is based on previous work with similar scope
| Example | |
Publication Costs We anticipate ~2-3 publications will be supported by this project in each year. Assuming an average cost of publishing in scientific journals of $500-750 per article, $1500/year are during each year of the award. Computer Software The data will be analyzed using SAS software. Funds of $500/year are requested to support the SAS licensing fee and for additional software costs (referencing, electronic art, etc). Animal Housing $40,250 is requested to support the maintenance and care of experimental animals for the 5-year duration of the award ($3,535 per year 1 – 2 and $11,060 per year 3 – 5 ). This includes housing at the Duke animal facility in MSRBII (based on the projected cage rate of $1.01/cage per diem). Calculations per year are outlined below. Year 1-2: We anticipate 20 cages for 25 weeks each, per year, for mouse experiments in specific aim 1. 20 cages x 1.01/cage/day x 175 days = $3,535/year Year 3-5: We anticipate 30 cages to maintain experimental mouse colonies in specific aim 2. 30 cages x 1.01/cage/day x 365 days = $11,060/year
Core Services $11,900 is requested to support the use of Duke Proteomics and Metabolomics Core Services in year 2 of this award. This includes analysis of immunoprecipitation samples by 90 minute gradient, using the Bradford assay, in-solution digestion, 90-min LC-MS/MS. As outlined in the proposed research strategy we anticipate 40 samples at the projected price of $595 per 2 samples. Cost calculations are based on price quote obtained from the Duke Proteomics and Metabolomics core.
|
Travel
- When budgeting travel that is directly related to your proposed research,
- Consider the destination, number of people traveling and duration of stay for all anticipated travel.
- Base travel cost calculations on institutional and NIH travel regulations, including advance notice, coach class airfare, reasonable hotel accommodations and per diem expenses per person and by trip. Travel regulations referenced above can be accessed via the following links;
- Also consider additional travel expenses, such as registration fees to attend a scientific conference, which may also also be included if directly related and required for the purpose of travel.
Note: Always refer to the FOA to determine if certain travel restrictions apply.
Travel: In the budget justification section;
1 Indicate the destination, number of travelers (and their project role. i.e. PI, postdoc etc) and duration of stay for all anticipated travel. |
Note:
- Travel support may only be requested for the Duke affiliated project personnel name on the application who are dedicating effort to the project.
- Be clear on whether travel is Domestic and/or International travel
Reminder:
- travel costs requested for subrecipients are indicated in the subaward budget and described in the justification provided separately.(see section below and subcontract agreement resource page for more information)
2. Clearly state how the travel is directly related to your proposed research. |
- Travel required to complete the proposed project activities (i.e., to data collection sites, in person training for project specific protocols)
- Travel to facilitate face-to-face meetings with collaborators (steering committee meetings)
- Travel to scientific conferences to present results and network with fellow investigators and collaborators in the field
3. Be clear on the frequency of travel. |
(i.e. annual meeting or conference where funding is requested per award year, vs. Protocol Training in year 1 and Steering committee meeting in year 3 of the award)
4. Provide an itemized breakdown of anticipated travel expenses, including advance notice, coach class airfare, hotel accommodations, per diem expenses and other travel expenses (registration fees etc). Cite adherence to institutional and NIH travel regulations. |
- Presenting the cost calculations in a table will add further clarification and make the justification easier to follow.
- For multiple trips and travelers, we recommend generating a table for each anticipated travel for simplicity.
- Round to the nearest dollar or 10 dollars to make the calculations easy to visually compute quickly
- Check your math. Make sure your calculations are correct and match the travel totals requested in the budget.
| Example |
Travel Funds will support travel to facilitate dissemination of research findings and face-to-face meetings between the PI and his collaborators at the Project Steering Committee Meeting and the annual American Society of Nephrology meeting. All travel cost estimates are based on institutional and NIH travel regulations, including advance notice, coach class airfare, reasonable hotel accommodations, and per diem expenses. For the PI to attend a 2-day Steering Committee Meeting, we assumed $400 airfare, $125 for ground transportation, 1 hotel night at $200/night, and $50/diem for 2 days for a total estimate of $825. For the PI to attend a 4-day ASN meeting, we assumed $500 airfare, $125 for ground transportation, 3 hotel nights at $200/night, and $50/diem for 4 days for a total estimate of $1425. Therefore, we have budgeted the following for travel and request $2,250 for PI travel support per each year of the award. |
Travel expense type | Cost per day | Increased per day | Subtotal | |
Airfare | $400 | n/a | $400 | |
Lodging | $200 | n/a | $200 | |
Meals/Incidentals | $50 | 2 days | $100 | |
Ground transport | $125 | n/a | $125 | |
Steering Committee Meeting Travel costs |
| $825 | ||
Travel expense type | Cost per day | Increased per day | Subtotal | |
Airfare | $500 | n/a | $500 | |
Lodging | $200 | 3 days | $600 | |
Meals/Incidentals | $50 | 4 days | $200 | |
Ground transport | $125 | n/a | $125 | |
ASN Meeting Travel costs | $1425 | |||
*The following specific budget items are excluded from the Modified Total Direct Cost (MTDC) base used to calculate overall project indirect costs (Duke F&A)
Equipment*
Clinical Research Considerations
For Clinical Research proposals it is imperative that you connect with the Nephrology Clinical Research Unit (CRU) early in the planning stages of the application. Their involvement, (in partner with the DoMRA, Nephrology grants & contracts team) will be crucial to preparing an accurate budget which will capture all project/study expenses necessary for implementing and completing a clinical research study at Duke University (and/or partnering hospitals/medical facilities etc.)
Important Note: It is important that you work with your CRU to review the structure of the study and identify the kind of space and resources that will be required to complete the study.
- Will lab space be required for the CRC to perform lab based protocols, processing of samples etc?
- (Applicable when the hospitals/clinics are not performing study related procedures or lab tests as part of Research Patient Care Cost expenses)
- What equipment will be required to perform specific study procedures? Are these resources available for use and located in the site where the work will be performed? Or will these need to be purchased. (ex. centrifuges, shakers, water baths, pipettes, etc)
- If sample collection is to occur, where and how will the samples be stored? Will cold storage equipment need to be purchased? (-80 Ultra low freezers, -20 freezers, 4C refrigerator, freezer (LN2))
- Will a temperature monitoring system be required for the unit according the sponsor or agency regulations, will this require installation of a new system?
Basic Science / Animal Research Considerations:
Determine what equipment may already be available to you through the Nephrology Division shared resources, Duke University Cores and other shared Resource facilities when considering when equipment purchases are necessary.
- If you request equipment that is already available (listed in the Facilities & Other Resources section or equipment page, for example) you must be able to explain why the current equipment is insufficient to accomplish the proposed research and how the new equipment’s use will be allocated specifically to the proposed research. Otherwise, NIH may disallow this cost.
- Reminder to new and junior Investigators. Reviewers expect you to need the basics when getting started. Do not assume you will be able to use another lab’s equipment – explicitly talk about your needs and requests upfront and early on before preparing and submitting an applicable.
Equipment Resources to Reference
- See a list of MSRBII Shared Resource Equipment for additional information on Nephrology shared equipment resources. Included are the equipment specifications, terms of use, lab location, and points of contact.
Refer also to Nephrologysignup.com to review the reservation platform, and access the frequency of use. Labs requiring high volumes of use should discuss this explicitly with the labs first, and consider purchasing additional equipment.
- Search core services available at Duke through MyResearchHome.
Refer to the list of available Core Services at Duke and register for an account through CoreResearch@Duke to search, reserve and use shared use equipment and facilities throughout Duke’s campus.
| *Equipment is excluded from the Modified Total Direct Cost (MTDC) base used to calculate overall project indirect costs (Duke F&A)* |
Equipment is defined as an item of property that has an acquisition cost of $5,000 or more and an expected service life of more than one year.
- Generally equipment is excluded from the F&A base, so if you have something with a short service life (< 1 year), even if it costs more than $5,000, you are better off including it under “supplies”.
- Please Note: General purpose equipment, such as desktop computers and laptops, that will be used on multiple projects or for personal use should not be listed as a direct cost , unless primarily or exclusively used in the actual conduct of the proposed scientific research.
- Equipment: In this section, provide a brief justification for each unit of equipment itemized in the budget. Each justification should include the following;
| 1. State the funds requested to purchase each unit of equipment |
- While the application does not require you to have a price quote for new equipment, including price quotes in your budget justification can aid in the evaluation of the equipment cost to support the project.
Note: This is typically only applicable when requesting funds for more complex pieces of equipment where reviewers may not be familiar with the expected price range, and/or when the cost of the unit appears particularly high. —-
- For standard units of lab / research equipment, (i.e. centrifuges, -80 freezers, cryofreezer etc) documented quotes etc. are not necessary to provide as part of the application. However, you should base your own cost estimations on the listed price of the model and unit proposed for purchase, and obtain quotes from the vendor.
| 2. Provide a brief justification for why the equipment is necessary to complete the project, how it will be used exclusively for the project during the award period |
- If you request equipment that is already available (listed in the Facilities & Other Resources section, for example), the narrative justification must explain why the current equipment is insufficient to accomplish the proposed research and how the new equipment’s use will be allocated specifically to the proposed research. Otherwise, NIH may disallow this cost.
| 3. Clearly state when in the project period the funds are requested (year 1, year 2, etc) and include a brief justification for the timing of the purchase. |
| Example – (AHA sample justification) |
Equipment Purchase of a Thermocycler ($10,000) and HPLC Fraction Collector ($15,000) is requested during the first year. The requested equipment is necessary for this project and will be used exclusively to analyse IV9-HLA-A-0201 complex. |
Patient Care Costs*
| Important Note: For Clinical Research proposals it is imperative that you connect with the Nephrology Clinical Research Unit (CRU) early in the planning stages of the application, particularly when planning your budget. |
| *Patient Care Costs are excluded from the Modified Total Direct Cost (MTDC) base used to calculate overall project indirect costs (Duke F&A)* |
Research Patient Care Costs – Applicable for clinical research proposals, expenses for this category include costs of any laboratory or clinical procedures that are conducted in an out-patient clinical or in-patient facility. The costs of these services normally are assigned to specific research projects through the development and application of research patient care rates
- Research Patients are defined as inpatient and outpatient subjects, volunteers, or donors participating in a research protocol
- Routine Services include regular room services, minor medical and surgical supplies, and the use of equipment and facilities, for which a separate charge is not customarily made.
- Ancillary Services are considered those special services for which charges are customarily made in addition to routine services, e.g., x-ray, operating room, laboratory, pharmacy, blood bank, and pathology.
Research Patient Care Costs: Few budgets contain patient care expenses, however if inpatient and/or outpatient costs are requested, the following information should be provided:
1 The names of any hospitals and/or clinics and the amounts requested for each. |
2. If both inpatient and outpatient costs are requested, provide information for each separately. |
3. Provide cost breakdown, number of days, number of patients, costs of tests/treatments. |
| 4. Justify the costs associated with standard care or research care. (Note: If these costs are associated with patient accrual, restrictions may be justified in the Notice of Award.) |
Refer to NIH Grants Policy Statement NIH Grants Policy Statement, Research Patient Care Costs
Additional budget items which are excluded from the Modified Total Direct Cost base include:
- Capital expenditures (construction and renovations)*
- Rental costs of off-site facilities*
- Tuition remission*
- Scholarships and fellowships*
** The portion of each subaward/subcontract in excess of $25,000 for the performance period of the project are excluded from the Modified Total Direct Cost (MTDC) base used to calculate overall project indirect costs (Duke F&A)
Consortium Subaward Costs*
Per the Duke ORA Pre-Award Manual – Chapter 1 Application Development –
Subrecipients
- Subrecipients have the same requirements to Duke as Duke has to the sponsor
- Duke must incorporate subaward / subcontract into prime application
- If the Sponsor follows the PHS FCOI policy (link to list of these sponsors), each subrecipient must provide the following for an application:
- Financial Conflict of Interest (FCOI) Form use- For Proposal or If Awarded
- Budget – Detailed SF424 (R&R) Budget form
- Budget Justification
- Scope of Work
- Document signed by subrecipient’s authorized institutional official
- Subrecipient may include their institution’s approved F&A rate in their budget (follow guidelines for how F&A rate is applied)
- All budget restrictions flow-down to the subrecipient’s budget
Subcontractor
The subcontractor will have their own justification of costs. However, a subcontract justification should be within your Duke justification.
Justification language should include:
- A summary of the scope of work
- Amount of total costs requested per year
- Justification of key personnel affiliated with the institution
Indirect Costs
Indirect Costs
Summary
Indirect costs should be considered costs which are not directly accountable within a sponsored research project, but can be considered in support of the research mission as a whole. Administration, security, lights, space, and libraries are some of the many things factored into indirect expenses.
Duke University negotiates their indirect rate with the federal government on an annual basis. Different activities merit different indirect cost percentages. Your main consideration is who is sponsoring the research, where the sponsored research will take place, and what type of sponsored research is being conducted.
What Happens If The Sponsor Does Not Cover My Indirect Rate?
Generally, Duke University requires that all sponsored projects use the appropriate federally-negotiated Facilities & Administrative (F&A) rate appropriate in budgetary calculations. However, the institution recognizes that some industry and foundation sponsors may have policies and terms and conditions that would create circumstances in which it would be appropriate to consider requests for reduced or waived F&A rates. In the event a reduction is being considered, consult your grants and contracts administrator.
Examples and Templates