Duke’s CTSA Responds Quickly to Dual Crises
In 2020, two events changed the world forever: the COVID-19 pandemic and the national reckoning of systemic racism. Both of these events served to highlight, among other things, the importance of programs like the Clinical and Translational Science Award (CTSA). As a far-reaching convener and resource within Duke and beyond, the CTSA program at Duke, managed by the CTSI, was called upon for coordination, resources, and leadership. Normal plans were upended with mandatory shutdowns of research laboratories, a sudden shift to remote working for nearly everyone, and a prioritization of COVID-related research. Immediate and unending attention to the issues of racial injustice is driving intrinsic changes in our personal, institutional, and cultural identities and practices. The Duke CTSA has supported all of these efforts and, in fact, has taken advantage of opportunities that were previously uncontemplated.
COVID-19 Response
The initial reaction to the pandemic at Duke was to identify people and resources that could be garnered to support COVID-19 related activities. The CTSI was deployed to serve as the clearinghouse for this information. Over a period of days, CTSI staff, working with University and School of Medicine leadership, disseminated a call to all researchers at Duke to identify specific projects, people, and resources that could be helpful to Duke’s COVID-19 response. Using MyResearchHome (a tool created by the CTSA — see figure below), the CTSI developed an online data collection tool, the COVID-19 Research & Activities Registry, and within one week, we had over 100 responses. The system currently includes more than 575 projects from across the University.
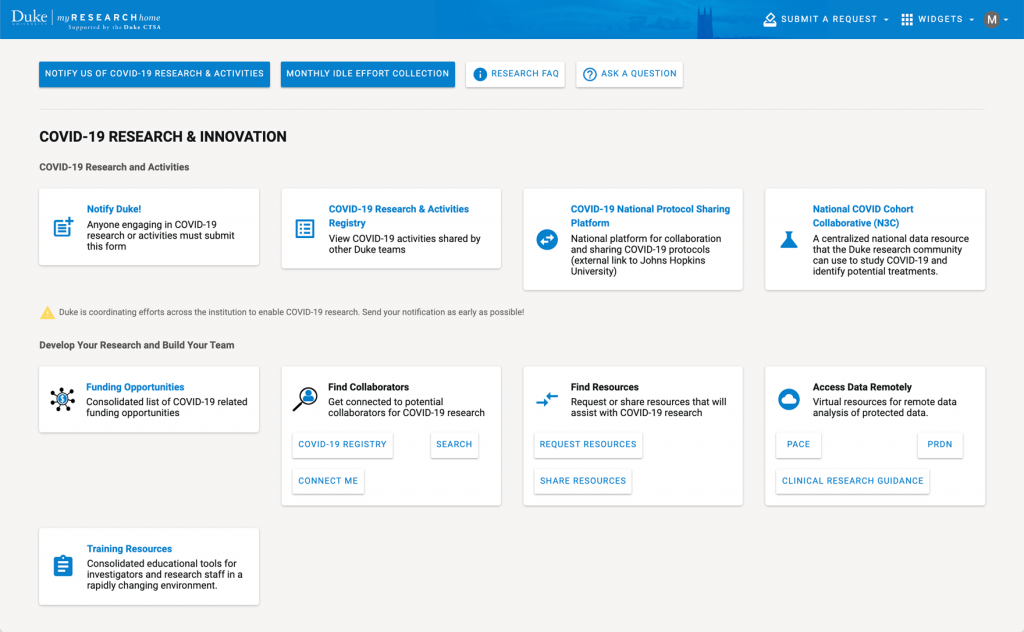
COVID Dashboard on My RESEARCH Home portal
In collaboration with School of Medicine leadership, the CTSA’s Participant and Interactions Core (Duke Office of Clinical Research) implemented a system of tiered criteria for ongoing research studies to evaluate direct patient benefit, and established processes for transitioning to remote activities. CTSA teams reviewed and approved “Return to Research” plans for all studies following a phased approach with approved guidelines and training for conducting research on-site. In addition, we created processes for COVID patient triage, to ensure equitable distribution of participants to COVID studies; remote monitoring requests; and research PPE distribution.
Other essential tools, processes, and resources developed or provided by the CTSA include:
- A symptoms reporting database for on-site employees across the Duke Health Enterprise (developed in collaboration with Duke University Health System and Duke Health Technology Solutions)
- A shared effort pool to reallocate available effort for research staff idled by suspended studies
- An intake and matching process for medical and graduate students to assist with COVID studies
- A grant “SWAT” team to assist with the rapid development of COVID-related research grants
- Staff support for the ACTT, Biorepository, and Community Watch studies at their inception
- Communications outreach related to COVID with the SoM through newsletters, website and Town Hall meetings
