Introduction
Over half of Australia’s 156 terrestrial snakes are venomous, making it the only continent with more venomous than non-venomous species (Williams, Wuster, & Fry, 2006). The most venomous land snake in the world, the inland taipan, is endemic to Australia (Australia Museum, 2014). Death adders, lowland copperheads, and red-bellied black snakes also inhabit the continent while sea snakes swim along its coasts. These snakes are all members of the family Elapidae, or the elapids.

The inland taipan, Oxyuranus scutellatus, is the world’s most venomous land snake.
Fixed Fangs, Venom, and Build
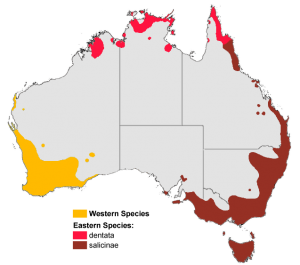
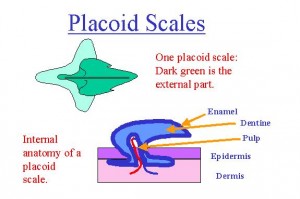
Elapids are characterized by a pair of hollow, fixed fangs that inject venom from the rear of the upper jaw into prey. The rostral location of the venom delivery apparatus differentiates elapids and sea snakes from other snakes. Usually, the venom of elapids is neurotoxic, but some species produce myotoxins, procoagulants, and anticoagulants. Most species are incapable of delivering medically significant bites to humans. Many Australian species remain unstudied, and their venom may become a resource for future drug development.
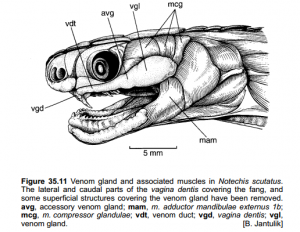
Image depicting a typical elapid’s fixed, hollow fangs and round pupils.
In general, elapids have smooth scales, slender bodies, and eyes with round pupils. Death adders are exceptions as some of these species are rough-scaled or cat-eyed (Shea, Shine, & Covacevich, 1993).
Species Diversity
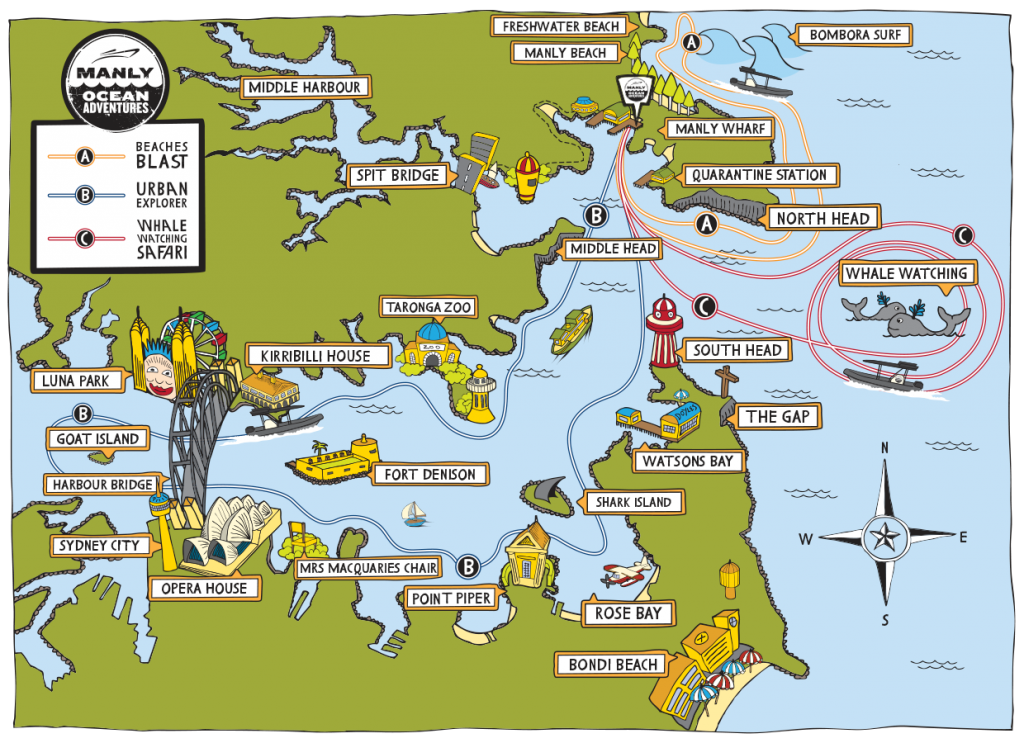
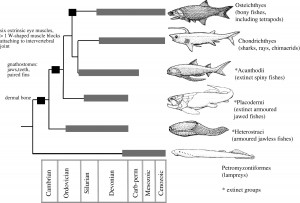
Roughly 300 species in 61 genera are currently recognized as elapids (Keogh, 1998). Found primarily in the tropics and subtropics of Asia, Australia, Africa, and the Americas, elapids consist of big and small, colorful and dull, and banded and solid individuals. They range in length from 18 centimeter Drysdalia, crowned snakes, to the 5.6 meter Ophiophagus hannah, king cobra (JRank, 2014). In the United States, the three species of native coral snake in the genera Micrurus and Micruroides are members of this family. These snakes are identified as venomous by the adage, “Red on yellow, kill a fellow.” Coral snakes in other parts of the world may be red and black, pink and blue, or have no banding at all (Szalay, 2014).
Cobras, a group of snakes found in Africa and Asia, are elapids that spread their necks into hoods when threatened. The four species of mamba in Africa in the genus Dendroaspis are diurnal elapids. The black mamba is one of the fastest snakes in the world; it slithers up to 20 kilometers per hour (National Geographic Society, 2014).
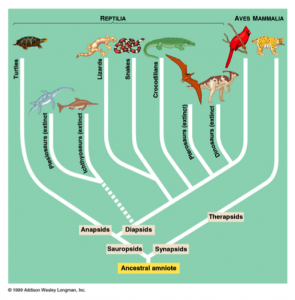
Of the seven families of snakes found in Australia, Elapidae is the biggest and most diverse (Phillips, Brown, & Shine, 2003). The genus Oxyuranus consists of the inland taipan, coastal taipan, and the central ranges taipan. The central ranges taipan was only discovered in the last decade through mitochondrial DNA analysis; this reveals the lack of exploration of remote areas in Australia and underscores the importance of genetic analysis in studying speciation (Doughty et al., 2007). Taipans feed on rats and small mammals, and their highly neurotoxic venom paralyzes their victims’ nervous system and causes blood-clotting. Notechis is the genus of Australia’s tiger snakes. These snakes give birth to 20 to 30 live young and received their common name from their alternating dark and light bands. Acanthophis are the death adders found in Australia and New Guinea. Death adders resemble other adders with their stocky builds and triangular-shaped heads, but they are not true vipers as they do not have long, hinged fangs. Their similarities are due to convergent evolution. Australian copperheads of the genus Austrelaps are also not pit vipers nor close relatives to Agkistrodon contortrix, the American copperhead (Australian Museum, 2014).
Evolutionary History
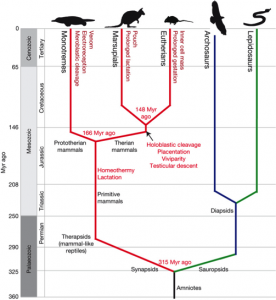
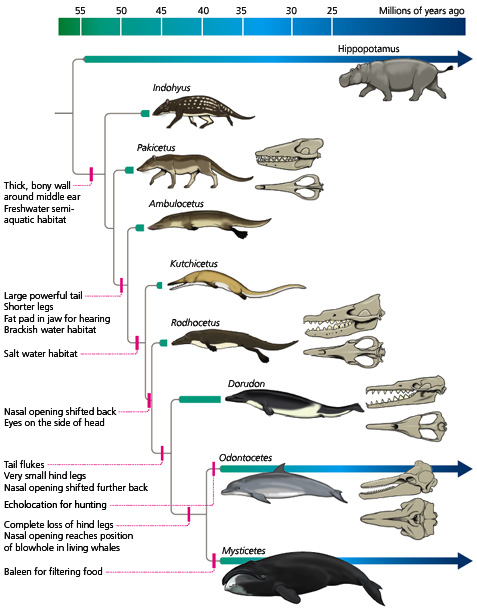
In the Cretaceous period (about 120 million years ago), it is speculated that the first snakes evolved from lizards that swam in the oceans or burrowed. The disappearance of limbs was a result of a modification of the Hox genes, the genes that control limb morphogenesis (Gilbert, 2000). Although the fossil record is poor, it is known that modern snakes diversified during the Paleocene (roughly 55 million years ago).

This marine lizard is a transitional fossil between lizards and snakes.
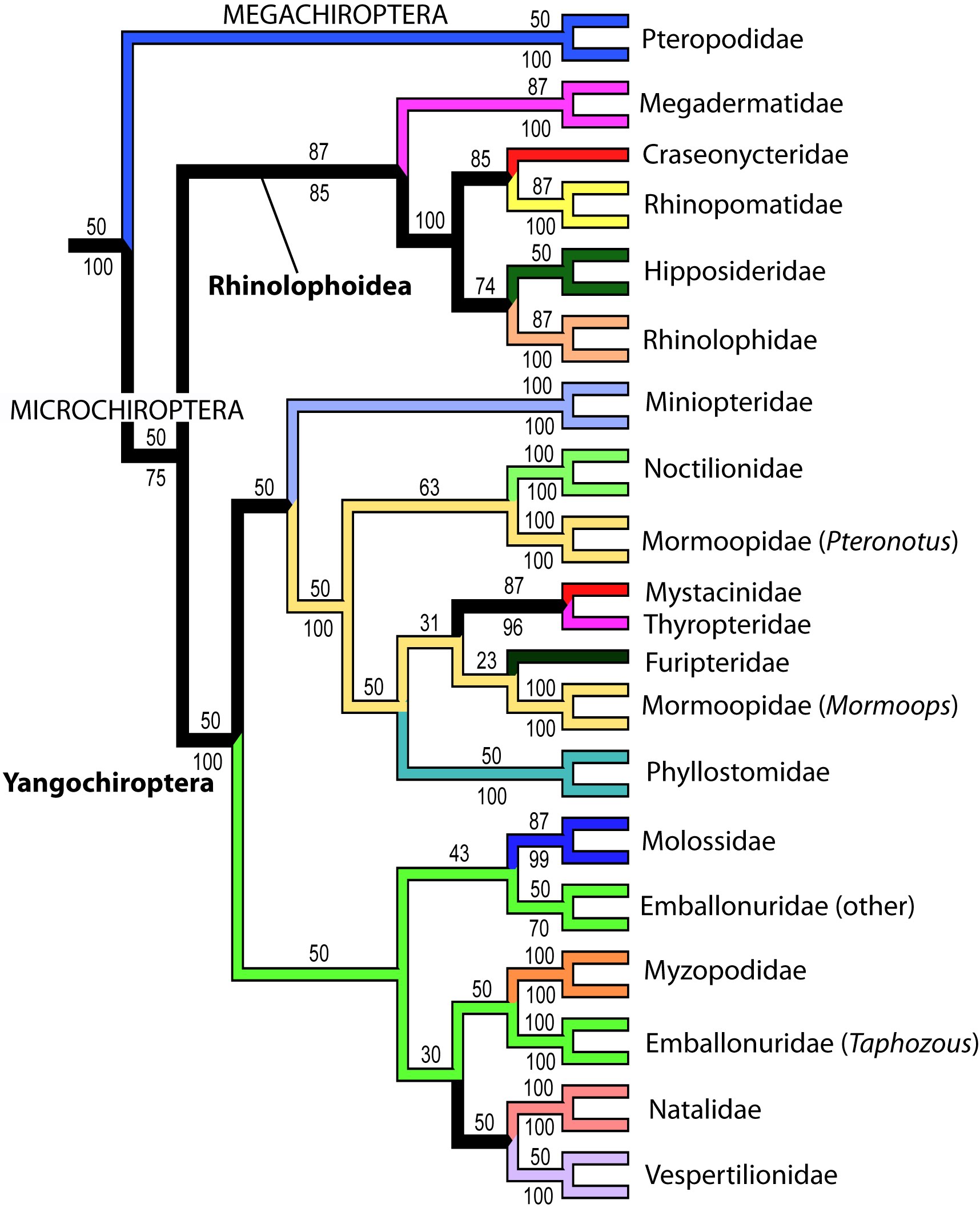
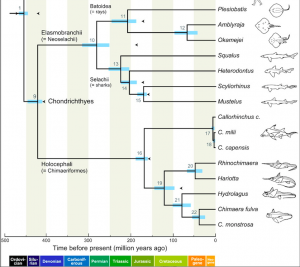
In the Miocene (approximately 20 million years ago), Australian members of Elapidae arrived from Asia. Upon their arrival, an adaptive radiation occurred in which elapids filled various ecological niches. This rapid evolution makes it difficult to determine the relationships between genera and species. However, it is generally agreed upon that the hydrophiines, the largest group of sea snakes, evolved from terrestrial Australian elapids 10 to 15 million years ago and radiated into 60 species. The Australian and Melanesian terrestrial elapids and sea kraits are also members of the sea snake subfamily Hydrophiinae. Their origin is Asian. Old world elapids found in Africa and Asia, like the cobras and mambas, belong to the subfamily Elapinae. It is suspected that their origin is Asian, African, or Afro-Asian. The Hydrophiinae and Elapinae combine to form the large family Elapidae (Keogh, 1998).
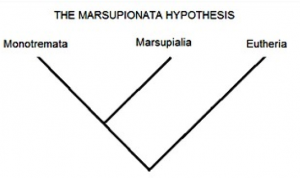
Two possible phylogenies depicting the relationship between terrestrial elapids and sea snakes.
Evidence of the land bridge between New Guinea and Australia during the Pleistocene and even the Holocene (1 million years ago to 10,000 years ago) is supported by the sharing of several elapid species, such as Furina tristis, the brown-headed snake, Oxyuranus scutellatus, the coastal taipan, Pseudechis australis, the common king brown, Rhinoplocephalus boschmai, the Carpentaria whip snake,and R. nigrostriatus, the Australian black-striped snake. When low precipitation and freezing lowered sea levels, these species were able to migrate north and south and vice versa (Shea, Shine, & Covacevich, 1993).
Ecology
Elapids mate in the autumn or spring, or sometimes both. Most elapids lay eggs (oviparous) although a few Australian species, such as the tiger and sea snakes, bear live young (ovoviviparous). Parental care for eggs or young has only been observed in king cobras. These snakes remain with their eggs to provide protection (JRank, 2014).
Depending on the species, terrestrial elapids may eat small mammals, birds, fishes, lizards, frogs, or other snakes. Some have specialized diets while others are less particular. North American coral snakes, for example, prefer dining on small lizards but will also eat frogs and other snakes (2014). Sea snakes munch on fishes, squids, or eels in coral reefs.
Elapids actively hunt, striking and biting their prey with their fangs. These fangs release venom that quickly slows the victim’s heartbeat, making it easier to eat. A unique adaptation of Australian death adders is to wriggle the tip of the tail in the air to fool prey into thinking a tasty grub is waiting to be eaten. When the prey gets close, the death adder strikes (Australian Museum, 2014).
Larger Australian elapids are generally diurnal, basking in the sun during warm days, while smaller Australian elapids are crepuscular or nocturnal, relying on transferred heat from objects and protected shelters for warmth. The activity of elapids varies by season. At the Top End, elapids are particularly active in the summer months when heavy precipitation occurs. In southern Australia, elapids may overwinter beneath superficial shelter and emerge during sunny, warm days (Shea, Shine, & Covacevich, 1993).
Elapids are suited to a variety of niches. Deserts, grasslands, woodlands, rainforests, and oceans make up a few of their habitats. Some elapids are found in rocky outcrops, like the Australian Hoplocephalus bungaroides, the broad-headed snake, while others have become specialized burrowers in arid environments, such as Ogmodon vitianus, the Fiji snake (Department of the Environment, 2014).
Three particularly hot spots for elapids exist in Australia: south-western Western Australia, south-eastern South Australia, and the region including eastern Queensland and extreme north-eastern New South Wales. These areas are rich in habitat diversity, providing elapids with more chances to specialize. Mixed vegetation thrives in the south-eastern Queensland region, and its subtropical climate supports animals adapted to both hot and cold weather (Shea, Shine, & Covacevich, 1993).
Conservation Status
Herpetofauna include some of the most imperiled species on Earth. A combination of toxins, global warming, disease, non-native predators, over collection, and habitat destruction contribute to their declining numbers (Center for Biological Diversity, 2014). In Australia, several terrestrial elapids are listed as vulnerable. The broad-headed snake faces fox and cat predation. In addition, its range covers the populated Sydney area in New South Wales, putting its habitat directly at risk of disturbance and development by humans. Furina dunmalli, Dunmall’s snake, has lost habitat from mining, agricultural activities, and the draining of swamps. It likes brushy, wooded areas, and fallen timber and ground litter have become difficult to find. Invasive predatory and plant species threaten this snake, too. Denisonia maculata, the ornamental snake, is listed as vulnerable as a result of land clearing and the cane toad (Department of the Environment, 2014). The toxins in the paratoid glands of this invasive toad are enough to kill some snakes that mistake them for tasty meals (Phillips, Brown, & Shine, 2003).
Unfortunately, water is not a safe haven for elapids, either. In Western Australia, Aipysurus apraefrontalis, short-nosed, and A. foliosquama, leaf-scaled, sea snakes are restricted to coral reefs. They were abundant in the Ashmore and Hibernia Reefs during the 1970s and have since mysteriously declined, although their habitats are intact. Nine other species of sea snake have disappeared from these reefs in the last fifteen years. Possible causes include sedimentation, changes in salinity, and air-gunning during seismic surveys for oil and gas (Sanders, 2013).
Increased awareness and education can help erase the negative attitudes many people harbor toward snakes. The majority of species are quite reclusive and shy, and respect is all that is required to appreciate snakes and stay safe. These animals fill ecological roles as predators, and conserving their habitat and understanding their biology is crucial for their continued survival.

Me happily holding a non-venomous smooth green snake. Snakes rock!
Works Cited
Australian Museum. 2014. Search the Site. Retrieved from http://australianmuseum.net.au
Center for Biological Diversity. 2014. The Amphibian and Reptile Extinction Crisis. Retrieved from http://www.biologicaldiversity.org/campaigns/amphibian_conservation/
Department of the Environment. 2014. Species Profile and Threats Database. Retrieved from http://www.environment.gov.au/sprat
Doughty et al. 2007. A New Species of Taipan (Elapidae: Oxyuranus) from Central Australia. Zootaxa, 1422: 45-58.
Gilbert, S. F. 2000. Hox Genes: Descent with Modification. In Developmental Biology Sixth Edition. U.S.
JRank. 2014. Kraits Cobras Sea Snakes and Relatives: Elapidae. Retrieved from Net Industries: http://animals.jrank.org/pages/3897/Cobras-Kraits-Sea-Snakes-Relatives-Elapidae.html
Keogh, J. S. 1998. Molecular Phylogeny of Elapid Snakes and a Consideration of Their Biogeographic History. Biological Journal of the Linnean Society, 63: 177-203.
National Geographic Society. 2014. Black Mamba. Retrieved from http://animals.nationalgeographic.com/animals/reptiles/black-mamba/?rptregcta=reg_free_np&rptregcampaign=20131016_rw_membership_r1p_intl_dr_w#
Phillips, B. L., Brown, G. P., & Shine, R. 2003. Assessing the Potential Impact of Cane Toads on Australian Snakes. Conservation Biology, 17: 1738-1747.
Sanders, K. 2013, February 21. Australian Endangered Species: Sea Snakes. The Conversation.
Shea, G., Shine, R., & Covacevich, J. 1993. Fauna of Australia: Family Elapidae. In G. J. Ross, P. L. Beesley, & C. G. Glasby, Volume 2A Amphibia and Reptilia. Canberra.
Szalay, J. 2014, March 7. Coral Sankes: Colors, Bites, Farts, and Facts. Retrieved from Live Science: http://www.livescience.com/43938-coral-snakes-colors-bites-farts-facts.html
Williams, D., Wuster, W., & Fry, B. G. 2006. The Good, the Bad, and the Ugly: Australian Snake Taxonomists and a History of the Taxonomy of Australia’s Venomous Snakes. Toxicon, 48: 919-930.
































































































































































































































































































 In case you just cringed, here’s a comforting thought: 3/4 of the mound is actually underground. That’s right, this is only 1/4 of their home. Oh wait…that just made it worse, didn’t it?
In case you just cringed, here’s a comforting thought: 3/4 of the mound is actually underground. That’s right, this is only 1/4 of their home. Oh wait…that just made it worse, didn’t it? We eventually left the termites to do what they do and moved on to Florence Falls for lunch. Lunch was a nice break from the termite talk, complete with Australian flavored potato chips (honey soy chicken?!?! What?!!?). But, what came after lunch was even better than termites AND food: swimming!
We eventually left the termites to do what they do and moved on to Florence Falls for lunch. Lunch was a nice break from the termite talk, complete with Australian flavored potato chips (honey soy chicken?!?! What?!!?). But, what came after lunch was even better than termites AND food: swimming! The water was pretty cold, but very refreshing. A few of us scrambled up 5-10 feet on the red, slippery rocks in between the two falls and took some pictures. Once we were up there, we were met with a small issue: climbing back down. The rocks were a little too slippery to go back the way we came, but a brave little 7 year old showed us how to slide off them, introducing us to the most natural/painful water slide I’ve ever had the pleasure of going down.
The water was pretty cold, but very refreshing. A few of us scrambled up 5-10 feet on the red, slippery rocks in between the two falls and took some pictures. Once we were up there, we were met with a small issue: climbing back down. The rocks were a little too slippery to go back the way we came, but a brave little 7 year old showed us how to slide off them, introducing us to the most natural/painful water slide I’ve ever had the pleasure of going down. They both survived. So, naturally, I had to do it too. Evan and I followed their path up the cliff and plunged into the water after them. To be perfectly honest, I thought the climb was much scarier than the jump, but that might just be the diver in me talking.
They both survived. So, naturally, I had to do it too. Evan and I followed their path up the cliff and plunged into the water after them. To be perfectly honest, I thought the climb was much scarier than the jump, but that might just be the diver in me talking. The few. The brave. The cliff-jumpers.
The few. The brave. The cliff-jumpers. And with that, we went back home to Alatai Apartments and crashed. Here’s to another exhausting, fun-filled day in Darwin!
And with that, we went back home to Alatai Apartments and crashed. Here’s to another exhausting, fun-filled day in Darwin!