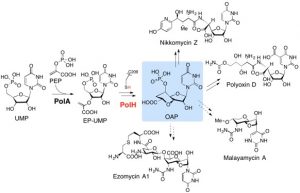
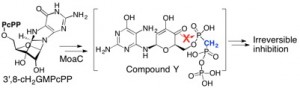
In this paper, we described detailed protocols of expression, purification, and characterization of MoaA and MoaC, as well as the isolation and characterization of 3′,8-cH2GTP. The in situ 13C NMR assay method was also described so that anyone interested can determine the product of MoaA without going through extensive purification in anaerobic glove box. We hope these protocols are informative to whoever interested in Moco biosynthesis or any other related systems.