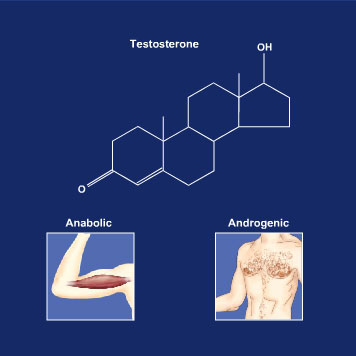
There are several problems with the use of testosterone. Since it has both androgenic1 effects (development of male sexual characteristics) and anabolic effects (promotion of muscle growth), both males and females may appear to be more “masculine.” Athletes have tried to get around this issue by using synthetic forms of testosterone that have a chemical structure modified slightly from the original testosterone. These synthetic versions are called anabolic steroids2 and the manufacturers claim that they are more selective in their ability to produce anabolic effects compared to androgenic effects. However, despite these claims, anabolic steroids do have androgenic (masculinizing) effects, and thus a new terminology has emerged – anabolic-androgenic steroids, or AAS. The androgenic effects of anabolic steroids are a big problem for females who can develop facial hair, male pattern baldness and deepening of the voice (some of these effects are irreversible!). Another problem with taking the natural form of testosterone is that it is not very effective when given orally. After oral administration, testosterone is absorbed from the intestine into the bloodstream, which takes it to the liver (see Module 1), where it is immediately metabolized (inactivated). Thus, relatively little testosterone circulates throughout the bloodstream to reach its target. To address this problem, the chemists have chemically modified the testosterone structure to make it more difficult for the liver to metabolize it. A major chemical modification is the addition of C and H atoms (alkyl group) on the 5-membered ring where the OH is bound; this is called carbon #17 (Figure 2). Thus, this modification allows more testosterone to be available in the general circulation. However, there is a problem. The addition of an alkyl group at the 17th C atom not only enables the testosterone to be more slowly metabolized by the liver, but it also causes the liver to work harder to get rid of it, eventually resulting in liver damage or cancer.
Figure 2 The structure of testosterone is shown. To increase the anabolic properties relative to the androgenic properties, chemists can modify the structure by adding alkyl groups (C and H atoms) to carbon #17 (where the OH bond is located).
Definitions:
1 a steroid hormone such as testosterone that is masculinizing (deepens voice, produces facial & chest hair, sperm production
2 synthetic versions of testosterone designed to promote muscle growth without producing androgenic effects. The better term is anabolic-androgenic steroid.
