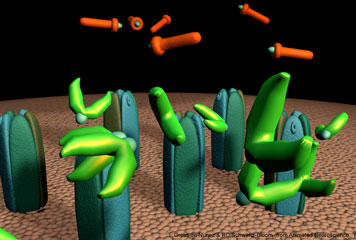
To bind to an enzyme1, receptor2 or transporter3, a drug4 must have a specific structure to “fit” into the protein. In addition, the protein exists in a conformation or 3-D shape that will allow bonds to form between the protein and the drug. For a drug to have an effect, it must be attracted to its target. Let’s use a receptor as the example for this discussion. The drug is attracted to its receptor by intermolecular forces. After these forces attract the drug to its receptor, they are also important in keeping it attached to the receptor for a sufficient period of time to initiate the biological changes within the organism. The forces that are important in the binding of drugs to receptors include, electrostatic attractions and van der Waals forces (e.g., hydrogen bonds5 and dipole-dipole forces) (Figure 9).
Figure 9 Watch how bonds form for different types of intermolecular forces that occur when a drug binds with its target. Although an example of a covalent bond is shown, it is quite rare in drug receptor-interactions.
A brief review of atomic structure will help in this discussion of the chemical bonds formed between drugs and their targets. The atom contains an internal nucleus with a positive electric charge. The nucleus is surrounded by electrons with a negative charge. Enough electrons are present to counterbalance the positive charge of the nucleus so that typically, the entire atom is electrically neutral. All atoms seek to reach chemical stability by giving up, taking on, or sharing electrons. This occurs in an atom-to-atom bond, (an intramolecular force) or between molecules (an intermolecular force). Intermolecular forces (between molecules) are the hallmark of drug-receptor interactions. A review of the types of intramolecular and intermolecular forces is found in the the next section.
Consider nicotine as an example for a drug-receptor interaction. Once the nicotine is in the body (bloodstream), it travels to tissues where it comes into contact with cells. The first process to occur in the binding of nicotine to the acetylcholine6 receptor is an electrostatic attraction between the nicotine and receptor molecules. The electrostatic force between oppositely charged atoms pulls the two molecules together from some distance away. All proteins are covalent molecules and most are polar7; they have a slightly positive charged region and a slightly negative charged region. (This depends on the particular amino acids that are present.) Most drugs also have a positive or negative charge. Look at the nicotine molecule in Figure 2. It can exist in its charged form when the N atoms form one more H bond (N atoms can form 4 bonds altogether). Although the electrostatic attraction has sufficient strength to allow the drug to interact with its receptor, the attraction is too weak to keep the nicotine bound to its receptor long enough to initiate a biological event – in this case the opening of an ion8 channel (Figure 7). So, as the nicotine and receptor molecules come closer together, van der Waals forces contribute to keeping the drug-receptor combination stable. Van der Waals forces are attractive forces between neutral atoms or groups. Although these are weak forces, they operate at close range and they are strongest when molecules are close together. One of the strongest types of van der Waals forces is the hydrogen bond. Hydrogen bonds occur between an H and two strongly negatively-charged groups (e.g., N, O, F). A single hydrogen bond is weaker than electrostatic forces, but when several hydrogen bonds occur simultaneously, they can increase the strength and stability of a drug-receptor interaction substantially. The van der Waals forces may seem insignificant because of their weak character, but actually, they provide the final critical component for the stability of the drug-receptor interaction. The combined effort of all of these forces allows the drug-receptor binding to be reversible. This is the most common situation for drug-target interactions and it is highly desirable!
In rare cases, covalent interactions can occur between a drug and its target. Covalent bonds are formed when a pair of electrons is shared between two atoms. For example, nerve gas9 has a phosphorus atom that reacts with an oxygen atom on the enzyme acetylcholinesterase10 (see Module 4). By sharing a pair of electrons, a new molecule is formed via a covalent interaction. The interaction is very strong, leading to irreversible binding between a drug and its target. This usually results in a sustained biological effect that cannot be altered. Clearly this would not be advantageous (imagine an overdose – how could we undo it?) and most drugs do not form covalent interactions with their targets. Sometimes we want an irreversible effect, such as in the case of penicillin or anti-cancer drugs, which have covalent interactions with bacterial enzymes or DNA, respectively.
Definitions:
1 a protein that catalyzes the rate at which a reaction occurs. It binds to one of the reactants (a substrate) to cause a change in the reactant’s structure, facilitating the reaction.
2 a protein to which hormones, neurotransmitters and drugs bind. They are usually located on cell membranes and elicit a function once bound.
3 a protein that usually exists within a membrane to transport a compound (either large or charged) across the membrane to the other side.
4 a substance that affects the structure or function of a cell or organism.
5 occurs between two strongly negatively charged ions. A type of Van der Waals force. When several occur simultaneously, they are responsible for increasing the stability of a drug-receptor interaction.
6 a neurotransmitter stored in vesicles of nerve terminals; it is found in neurons within the central nervous system, the somatic nervous system, the parasympathetic nervous system and the sympathetic nervous system.
7 a chemical property of a substance that indicates an uneven distribution of charge within the molecule. A polar substance or drug mixes well with water but not with organic solvents and lipids. Polar or charged compounds do not cross cell membranes (lipid) very easily.
8 an atom, radical, or molecule that has gained or lost one or more electrons. Therefore it acquires a net negative or positive charge.
9 a group of very lipophilic compounds (e.g. sarin, tabun, soman) that can exist as a vapor at room temperature. They contain phosphorus groups and bind avidly to acetylcholinesterase to inhibit its activity. The inhibition of acetylcholinesterase causes the accumulation of acetylcholine in all areas of the nervous system, causing excessive muscle contraction followed by paralysis, secretions, seizures and death by respiratory failure.
10 the enzyme that facilitates the hydrolysis (by water) of acetylcholine into choline and acetic acid. It is found near neurons that release acetylcholine.
Figure 2 The chemical structure is shown for several alkaloids found in plants. Alkaloids contain a nitrogen atom, which gives the compound a basic structure. THS is not an alkaloid – note that it doesn’t contain a N atom.
Figure 7 Nicotine binds to the acetylcholine receptor, triggering the influx of Na+ into the nerve or muscle cell. From: Animated Neuroscience & the Actions of Nicotine, Cocaine and Marijuana in the Brain (Gross de Nunez & Schwartz-Bloom).