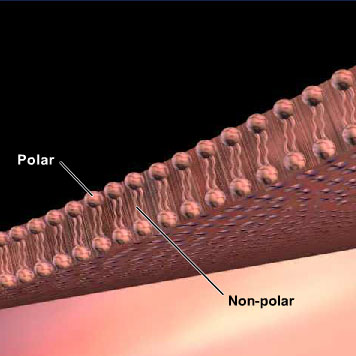
The ability of drugs1 or other molecules to pass through cell membranes is based on 1) the characteristics of the membrane and 2) the physiochemical characteristics of the drug. The membrane is a sandwich (bilayer) of lipids, with the polar2 or hydrophilic3 (water-loving) headgroups arranged at the surfaces of the membrane and the non-polar4 or hydrophobic5 (water-fearing) fatty acid carbon chains in the middle (see Figure 2). Drugs that are unionized and non-polar are able to pass through the membrane easily because they dissolve in the hydrophobic core of the membrane. They use the driving force of the concentration gradient to move from the side of higher concentration to the side of lower concentration, until an equilibrium is reached (passive diffusion6). Thus, cocaine passes through membranes readily by passive diffusion when it is in its unionized or free base form. Even if the ionized7 form is administered (i.e., by snorting8 or injecting) it is quickly converted to the unionized form at the normal physiological pH of 7.4 (in blood and tissues). Cocaine is a weak base9, so it has less tendency to ionize at a neutral pH compared to a more acidic environment. [This is indicated by the high dissociation constant or pKa (~8.7) for cocaine listed in chemistry handbooks and other reference books.]. Although membranes are hydrophobic in nature, there are small gaps between cells of the membranes through which water passes. Any compound dissolved in water (this means it is charged) that is small enough, i.e. less than a molecular weight of 100 daltons, can pass through the gaps with the concentration gradient.
Figure 2 Schematic view of a cell membrane. Lipids are arranged with polar head-groups facing the outside and inside of the cell, while the fatty acid chains form the non-polar (hydrophobic) membrane interior.
Capillary membranes are a special case. Capillaries (made up of endothelial cells) have numerous pores (“fenestrae” – latin for windows). These pores are actually spaces within the endothelial cells and they are larger than the small pores found in non-capillary membranes. The fenestrae allow large molecules (up to molecular weights of 25,000 daltons) and charged molecules to pass through without difficulty (Figure 3). So capillaries are much less restrictive to the passage of solutes. This property allows large molecules such as proteins and water-soluble vitamins to be delivered to other cells throughout the body.
Figure 3 Cross section of capillary showing endothelial cells. In the non-brain capillary fenestrae are present. In brain capillaries the endothelial cells are tightly packed and no fenestrae are present.
Definitions:
1 A substance that affects the structure or function of a cell or organism.
2 A chemical property of a substance that indicates an uneven distribution of charge within the molecule. A polar substance or drug mixes well with water, but not with organic solvents and lipids. Polar or charged compounds do not cross cell membranes (lipid) very easily.
3 Dissolves readily in water. Hydrophilic compounds exist in an ionized or polar form and have difficulty crossing biological membranes (except capillary membranes).
4 A chemical property of a substance that indicates an even distribution of charge within the molecule. A non-polar or non-charged compound mixes well with organic solvents and lipids but not with water.
5 “Water fearing”; a compound that is soluble in fat but not water. This is typical of compounds with chains of C atoms.
6 The movement of a solute in its uncharged form to cross a membrane along a concentration gradient. No energy is required.
7 An atom, radical, or molecule that has gained or lost one or more electrons. Therefore it acquires a net negative or positive charge.
8 To breathe in a compound in solid form through the nostrils. With reference to cocaine, it is the hydrochloride salt.
9 A compound that tends to accept a H+ when placed in an acidic solution.