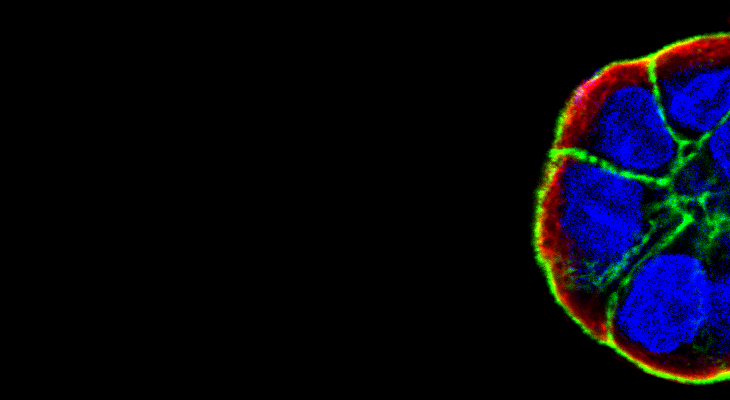
Establishment and maintenance of epithelial cell polarity is regulated in part by signaling from adhesion receptors. Loss of cell polarity is associated with multiple pathologies including the initiation and progression of various cancers. The β1-integrin plays a role in the regulation of cell polarity. We identified a novel role for the Arg (Abl2) kinase in the regulation of β1-integrin signals and epithelial cell polarity. In a three-dimensional (3D) cell culture model, activation of Arg resulted in a striking inversion of apical-basal polarity (Figure 1).
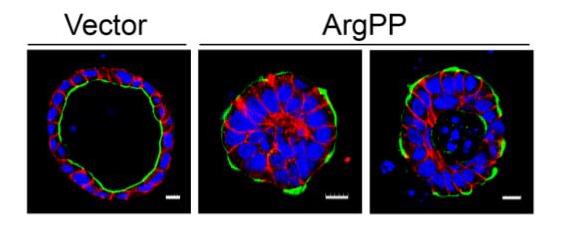
Fig. 1 – MDCKII epithelial cell expressing either empty vector or constitutively active Arg (ArgPP) were grown in collagen gels for 12 days. Cysts were stained for the apical marker gp135 (green), the adherens junction marker E-cadherin (red) and DAPI (blue). Scale bars represent 10 mm.
In contrast, loss of Arg function impaired the establishment of a polarized epithelial cyst structure. Active Arg kinase disrupted β1-integrin signaling and localization, and inhibited Rac1-mediated laminin assembly. Disruption of β1-integrin function by active Arg altered the distribution of selected polarity proteins through a Rap1 GTPase pathway. We showed that Rap1 and Rac1 signal independently in the regulation of epithelial polarity downstream of the Arg kinase. Thus, abnormal and persistent Arg activation may drive loss of epithelial cell polarity in cancer and other pathologies (Li and Pendergast, 2011).