ANTIMICROBIAL PEPTIDES: Investigation of antimicrobial peptides that target Lipid II
For decades, antimicrobial chemotherapies have been utilized successfully for the treatment of infectious disease. However, cases of widespread antibiotic resistance and recent emergence of the CDC's 'rugent threat' status pathogens such as methicillin resisant S. aureus (MRSA), vancomycin resistant Enterococci (VRE), and Clostridium difficile have highlighted a need for new antimicrobial drug discovery efforts.
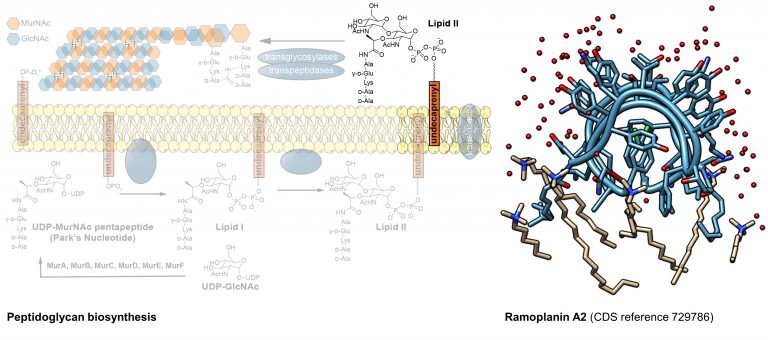
Some of the most successful antimicrobial classes target precursors of cell wall biosynthesis. Antimicrobial peptides such as the ramoplanin family of antibiotics as well as certain members of the calcium-dependent antibiotics and lasso peptides inhibit Gram-positive pathogens through selective binding to Lipid II, a key late stage intermediate in cell wall biosynthesis. Sequestration of Lipid II disrupts peptidoglycan polymerization, resulting in a weakened cell wall and ultimately cell death. Elucidation of the binding interactions at the antibiotic:Lipid II interface is essential for understanding the potent activity of these antibiotics, as well as for the rational design of new derivatives. Our lab focuses on developing methods for synthetic and biosynthetic access to these peptides in order to investigate their mechanisms of substrate recognition and bioactivity using crystallography and NMR techniques.