By Ashley Yeager
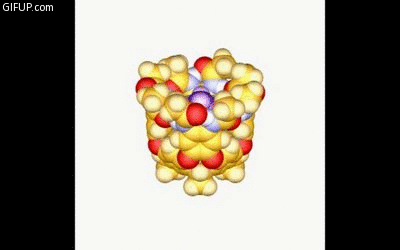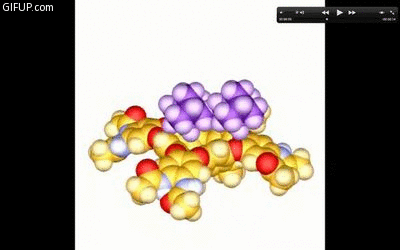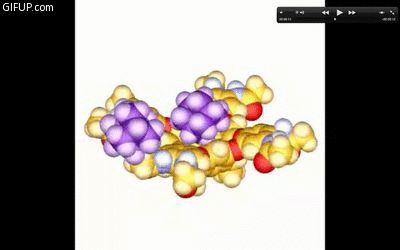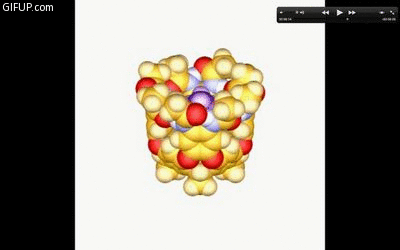
A new guest molecule knocks out the captive one in a cavitand just before it snaps shut. Credit: Lubomir Sebo.
Kids know when they’re going to get a tasty treat like M&Ms. They hold out their hands, palm up, and the snap their fingers around the chocolaty treats like a venus fly trap around a fly.
About a decade ago, Julius Rebek of the Scripps Research Institute in La Jolla, Calif., and his collaborators created a molecule that could do the exact same thing, trapping other molecules in much the same way.
Rebek’s goals was to understand how molecules behave when confined to small spaces. To do this, the chemists created a molecule called a cavitand, which self-assembles through bonds of hydrogen atoms into hand-like structures.
During an April 11 chemistry seminar, Rebek described how the walls or “fingers” of a cavitand use strong hydrogen bonds to curl around and snare another molecule. As a result, prying the cavitand open is a lot like trying to take candy from the closed grip of a child. It can be done but it takes energy and a bit of coaxing.
Rebek said that the cavitands’ finger-based hydrogen bonds can rupture. In response, the molecule then acts like a kid who opens his hand to show his M&Ms to a sibling, tempting her to take some. The captive molecule in the cavitand is exposed, and another can come in and knock it out of place, like a sister throwing something into her brother’s hand to knock out M&Ms for herself. Both the hand and the cavitand clasp shut quickly, taking a new type of treasure into their clutches.
By designing cavitands and other self-assembling molecular traps, chemists have begun to explore the way acids bind when trapped, how to control nano-sized spaces and how to switch molecules in and out of these nanospaces. The discoveries, Rebek said, will help scientists understand the binding and movement of molecules and the nanospaces where life’s most fundamental chemical reactions occur.
