The chemical reaction inside the Breathalyzer™ includes both oxidation and reduction. The Breathalyzer™ contains a chamber with several compounds to support these reactions. They include:
- Potassium dichromate
- Sulfuric acid
- Silver nitrate
When the potassium dichromate solution in the Breathalyzer™ reacts with ethanol, the potassium dichromate loses an oxygen atom.
- This process is called reduction–when a compound loses oxygen, gains hydrogen, or gains (partially gains) electrons.
- The reduction converts orange potassium dichromate into a green solution containing chromium sulfate.
At the same time dichromate ion gets reduced to chromium ion, ethanol gets oxidized to acetic acid. Oxidation reactions often occur simultaneously with reduction reactions and are commonly abbreviated as redox reactions.
- Oxidation occurs when an element combines with oxygen to give an oxide. For example, the oxide of hydrogen is water.
- Oxidation is the gain of oxygen, the loss of hydrogen, or the loss (or partial loss) of electrons
Silver nitrate serves as a catalyst for the reaction to increase the rate at which the dichromate gets reduced.
Sulfuric acid in the test chamber helps to remove the alcohol from the exhaled air into the test solution and to provide the necessary acidic conditions.
Oxidation and reduction (redox) reactions are opposing reactions that occur simultaneously.
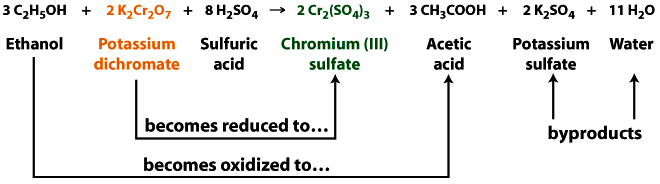 Figure 4.9 This is the balanced equation to show how ethanol is oxidized to acetic acid
Figure 4.9 This is the balanced equation to show how ethanol is oxidized to acetic acid
Learn more about redox reactions.