With sufficient kinetic energy, most liquids can become gases through the process of vaporization. Whether or not a liquid will vaporize depends upon the chemical properties of the liquid. Volatile liquids, including ethanol, vaporize with relative ease. Scientists commonly use the boiling point of a liquid as the measure of volatility.
- Volatile liquids have low boiling points.
- A liquid with a low boiling point will begin to boil faster than liquids with higher boiling points.
- Much less energy (in the form of heat) is required to break the intermolecular bonds of a volatile liquid than those of liquids having higher boiling points.
- Once enough energy is supplied to break apart the bonds between molecules, the molecules are free to expand and escape the liquid surface in the form of a gas.
Chemical Bonds contribute to volatility
The major attractive forces between molecules in a liquid are called hydrogen bonds. Less hydrogen bonding is expected between molecules of a volatile liquid compared with other less volatile liquids. With fewer hydrogen bonds holding volatile compounds in the liquid state, only minimal energy is required to break the bonds and allow the molecules to drift apart and escape from the surface of the liquid as a gas.
Review the properties of different chemical bonds.
The transition of a liquid to a gas is called vaporization. The reverse reaction, converting a gas to a liquid is called condensation.
- In a partially filled container, liquid particles will escape the surface and vaporize the air above the liquid.
- As gas concentrations accumulate in the air, the distance separating individual molecules decreases until Van der Waals and hydrogen bonds can drive clusters of molecules back into liquid form (condensation).
- Eventually, vaporization and condensation reach a state of equilibrium-no particles are lost, instead the gas phase is constantly being recycled into the liquid phase (think about a simmering pot of water with a lid on).
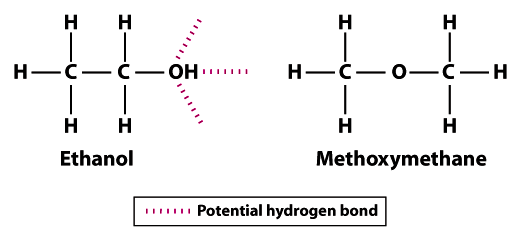 The structures show why ethanol is less volatile than methoxymethane; ethanol can form three hydrogen bonds and methoxymethane can’t form any.Note that the hydrogen atoms in methoxymethane cannot participate in hydrogen bonding with neighboring oxygen atoms. The hydrogen atom of the hydroxyl group (OH) in ethanol increases the potential for hydrogen bonding between neighboring ethanol molecules. Compared to methoxymethane, ethanol is not nearly as volatile. The boiling point of 78.5°C for ethanol is significantly higher compared with -24.8°C for methoxymethane. This example illustrates the significance of bond strength in general and hydrogen bonding specifically as a determinant of volatility of a molecule.
The structures show why ethanol is less volatile than methoxymethane; ethanol can form three hydrogen bonds and methoxymethane can’t form any.Note that the hydrogen atoms in methoxymethane cannot participate in hydrogen bonding with neighboring oxygen atoms. The hydrogen atom of the hydroxyl group (OH) in ethanol increases the potential for hydrogen bonding between neighboring ethanol molecules. Compared to methoxymethane, ethanol is not nearly as volatile. The boiling point of 78.5°C for ethanol is significantly higher compared with -24.8°C for methoxymethane. This example illustrates the significance of bond strength in general and hydrogen bonding specifically as a determinant of volatility of a molecule.