To understand the functions and mechanisms of enzymes, we use a variety of techniques. Following is a brief list of techniques that we have used. Each student/postdoc will learn many of these techniques, providing comprehensive research opportunities.
Enzyme kinetics: Kinetics of enzyme-catalyzed reactions are essential to understanding the function and mechanism of enzymes. We have extensive experience in steady-state and pre-steady state kinetics, such as stopped-flow and rapid quenching techniques. We use these methods in combination with the other biochemical and biophysical techniques.


Protein preparation: Protein preparation is central to all of our projects. We have extensive skills in the preparation of various proteins, including metalloenzymes that require anaerobic techniques, and membrane proteins that require detergent solubilization. We also use various heterologous protein expression systems to purify these challenging proteins.

Small molecule and carbohydrate characterization: Quantitative characterization of enzyme reaction products is central to our study. Therefore, we use a variety of analytical methodologies, including NMR, HPLC, MS, SEC (size exclusion chromatography), and HPAEC-PAD (high-performance anion-exchange chromatography coupled with pulsed amperometric detection).
Chemoenzymatic synthesis of small molecules: In many of our projects, we use small molecules as probes to characterize the enzymes of interest. These small-molecule probes are synthesized in the lab using a combination of chemical and enzymatic approaches, which provide us significant flexibility.
Biophysical spectroscopy:
Electron paramagnetic resonance spectroscopy (EPR): Many of our target enzymes use redox-active metals and organic free radicals. Characterization of these paramagnetic species is critical to understand their functions in enzyme catalysis. EPR spectroscopy is a powerful approach to characterize the paramagnetic species in biological samples quantitatively.

Fluorescent microscope: Some of our questions, such as the mode of action of antifungal drugs, require studies in intact cells. In these cases, we use fluorescent microscopy combined with fluorescently labeled probes.
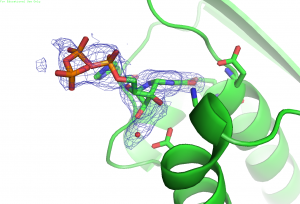
Structural biology: Protein structures are necessary to understand the mechanism and function of enzymes. We have ongoing collaborations with X-ray structural biologists within and outside the department (see PNAS 2015).